1. Introduction
In March 2022, Kyowa Hakko Bio Company Ltd, Japan (“the applicant”) submitted a full novel food application for the authorisation of 3’-sialyllactose (3’-SL) sodium salt. The novel food is a water soluble white to off-white powder composed of ≥ 82.0% w/w dry matter (DM) of 3’-SL sodium salt, which is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli W. 3’-SL sodium salt is intended to be used as a source of human identical milk oligosaccharide.
The FSA and FSS have undertaken a safety assessment for 3’-SL sodium salt under the novel foods legislation, assimilated Regulation (EU) 2015/2283. To support the safety assessment, the ACNFP provided the advice outlined in this opinion to the FSA and FSS.
The evaluation by the ACNFP assessed the food safety risks of the novel food and its production, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Agency (EFSA) for full novel food applications is retained as the basis and structure for the assessment (EFSA NDA Panel, 2021).
Following the review by the ACNFP in September 2023, further information was requested from the applicant concerning the identity, the production process, the compositional information, the stability, the nutritional information, toxicological information, and allergenicity information on 3’-SL sodium salt, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 165th meeting, allowing the FSA and FSS to complete the risk assessment.
The document outlines the conclusions of the FSA and FSS on the safety of 3’-SL sodium salt as a novel food.
2. Assessment
2.1. Identity of the novel food
The novel food is a white to off-white powder which is mainly composed of 3’-SL sodium salt (≥ 82% w/w DM). Other saccharides are present in smaller quantities: sialic acid (≤ 6.0% w/w DM), D-glucose (≤ 3.0% w/w DM), D-lactose (≤ 3.0% w/w DM), 3’-sialyllactulose and 6’-SL sodium salt (≤ 5.0% w/w DM, sum of both) and a small fraction of other related saccharides (sum of other carbohydrates ≤ 12.0% w/w DM).
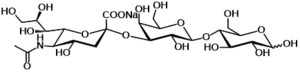
3’-SL is a trisaccharide consisting of D-glucose, D-galactose, and sialic acid (N-acetyl neuraminic acid) (see figure 1). This is identical to the structure of 3’-SL in human breast milk.
3’-SL sodium salt is classified as a purified chemical substance and characterised by the following information:
-
IUPAC name sodium; (2S, 4S, 5R, 6R)-5-acetamido-2-[(2R, 3S, 4S,
5R, 6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-[(2R, 3S, 4R,
5R)-4, 5, 6-trihydroxy-2-(hydroxymethyl)oxan-3-
yl]oxyoxan-4-yl]oxy-4-hydroxy-6-[(1R, 2R)-1, 2, 3-
trihydroxypropyl]oxane-2-carboxylate -
CAS number 128596-80-5
-
Molecular weight 655.53 g/mol
-
Molecular formula C23H38NO19Na
The structure of 3’-SL sodium salt in the novel food was confirmed using liquid chromatography – tandem mass spectrometry (LC–MS/MS) and nuclear magnetic resonance (NMR) spectroscopy: 1H-NMR, 13C-NMR, and two-dimensional (2D) NMR studies including COSY (correlation spectroscopy), TOCSY (total correlation spectroscopy), HETCOR (heteronuclear correlation) and HMBC (heteronuclear multiple bond correlation) methodologies.
2D-NMR analysis using high purity standards of 3’-SL sodium salt and 6’-SL sodium salt, a conformational isomer of 3’-SL sodium salt, demonstrated that the sialic acid is linked to D-galactose by α-(2"-3’) bonds. This provides unequivocal evidence on the structure of 3’-SL sodium salt.
High-performance liquid chromatography – charged aerosol detection (HPLC-CAD) was used to characterise 3’-SL sodium salt in eight batches of the novel food – five batches manufactured using soy peptone in the fermentation media and three batches manufactured without soy peptone in the fermentation media.
2.2. Production Process
The production microorganism, E. coli NEO3, used to manufacture the novel food is a genetically modified derivative of E. coli W (Waksman’s strain) that functions as a processing aid as defined in Article 3(2)(b) of assimilated Regulation (EC) No.1333/2008 on food additives. A novel food produced by a GMO does not fall under the remit of the GMO legislation, assimilated Regulation (EC) No 1829/2003 or assimilated Regulation (EC) No 1830/2003, when the production microorganism is removed during the manufacturing process and therefore no recombinant DNA remains. This has been confirmed in the compositional analysis as detailed below.
The novel food is classified as category 1 under the EFSA GMO guidance: chemically defined purified compounds and their mixtures in which both genetically modified microorganisms (GMMs) and newly introduced genes have been removed, under EFSA guidance, which categorises GMMs and their products for risk assessment purposes (FSA GMO Panel, 2011), which the FSA have retained for the purposes of technical review.
Although E. coli is not considered to be suitable for qualified presumption of safety (QPS) status (EFSA BIOHAZ Panel, 2023), E. coli W is widely used for biotechnological applications. Genomic analysis confirms that the genes required for pathogenicity are missing key components or they have been mutationally inactivated (Archer et al., 2011). Furthermore, E. coli W is considered to be a safe and non-pathogenic microorganism because this does not cause disease in healthy adult humans or colonise the human gut (Bauer et al., 2008; NIH, 2019). On the basis of this information, the new production strain organism does not introduce any new risks that need to be evaluated and managed.
The absence of bacteria from the Enterobacteriaceae family (ISO 21528-1:2017) and residual bacterial DNA (LOQ = 4 µg/kg) confirms the genetically modified E. coli NEO3 is not present in the novel food.
The first stage of the production process involves the conversion of D-lactose and D-glucose to 3’-SL by the adapted cellular metabolism of the production microorganism (The commercial production process does not use soy peptone). Glucose acts as an exclusive energy and carbon source, and lactose as a substrate for the biosynthesis of 3’-SL. The 3’-SL is released from the E. coli NEO3 into the fermentation broth. The production microorganism, E. coli NEO3, is removed from the culture medium by microfiltration at the end of the fermentation process.
The second stage of the production process involves a series of purification and isolation steps (filtration, ion exchange, concentration and spray drying) to the obtain the final purified novel food in powder form.
Information on the acceptance criteria for the raw materials and processing aids was provided. The purity criteria for the reagents used in the manufacture of 3’-SL sodium salt are listed in Table 1.
The novel food is produced in line with Hazard Analysis and Critical Control Point (HACCP) principles. The manufacturing facility is FSSC (Food Safety System Certification) 22000 certified.
Bacterial contamination of the novel food is controlled by monitoring the purification steps and the membrane filtration step prior to spray drying. To prevent bacterial growth, the water content in the finished product is monitored and specified at ≤ 10.5% w/w. Endotoxin levels in the novel food are controlled by ultrafiltration and compliance with the specified level at ≤ 10 EU/mg.
The production process has characterised the potential hazards and the corresponding control measures are appropriate.
2.3. Compositional information
Results from five independent batches of 3’-SL sodium salt manufactured using fermentation media containing soy peptone and three independent batches of 3’-SL sodium salt manufactured using fermentation media without soy peptone (commercial production process). These results demonstrate that the novel food is appropriately characterised (Table 2).
The composition data demonstrates that the novel food is consistently the same when the manufactured with or without soya peptone in the fermentation media.
Sialic acid, glucose, lactose, 3’-sialyllacultose, and 6’-SL sodium salt are naturally occurring components of human milk. Glucose is a breakdown product of the naturally occurring milk sugar lactose and 3’-sialyllactulose, is an isomerisation product of 3’-SL sodium salt, formed when the terminal glucose moiety isomerizes into fructose (EFSA NDA Panel, 2020).
An assessment was conducted in accordance with the EFSA Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles in the novel food (EFSA, 2021). A solubility in water test was conducted in two batches of the novel food produced with soy peptone and three batches without soy peptone in the fermentation media. The results confirmed that the novel food exceeded the decision criteria for solubility (> 33.3 g/L) as described in the guidance. Therefore, further evaluation for the presence of nanoparticles in the novel food is not required.
Certification was provided to demonstrate that the contract laboratories were accredited to perform these analytical studies. Where in-house analysis was utilised, full methodology and supporting validation documentation was provided.
The data presented indicate the novel food and any hazards present were appropriately characterised.
2.4. Stability
Results from an ongoing 36-month real-time stability study (25 ± 2°C; 60 ± 5% Relative Humidity) on a single batch of novel food produced with soy peptone in the fermentation media was provided. Data covering 24 months was reported for 3’-SL. Testing for, carbohydrate, water content, physicochemical parameters, and water activity was undertaken at the 6- and 12-month time-points. These endpoints in addition to microbiology quality were assessed at 24 months. No significant changes were observed, and microbial parameters were below the limits of detection.
A 6-month accelerated stability study (40 ± 2°C; 75 ± 5% Relative Humidity) was conducted on five batches of novel food produced with soy peptone in the fermentation media. Data for the following parameters was provided: 3’-SL sodium salt, carbohydrate, sodium, water content, and physicochemical parameters and water activity. No significant changes were observed.
No stability data was provided for the novel food in different food matrices.
The presence of soy peptone in the fermentation media is not expected to have a significant impact on the composition of the novel food. On this basis, the data provided supports the stability of the novel food, manufactured with or without soy peptone in the fermentation media, for up to 24 months.
2.5. Specification
The specification parameters for the novel food (Table 3) were assessed using internationally recognised methods or are otherwise determined using internally developed and validated methods.
The information provided is sufficient for the specification of the novel food, and appropriately characterises the novel food seeking authorisation.
2.6. History of Use
There is no history of use for the novel food in the UK.
3’-SL sodium salt, which is a major constituent of the novel food, has been authorised in EU (assimilated Commission Implementing Regulation (EU) 2021/96 and assimilated Commission Implementing Regulation (EU) 2023/113) for use in foods and food supplements. The authorised 3’-SL sodium salt is produced by fermentation using a genetically modified strains of E. coli K-12 DH1 and E. coli BL21 (DE3), respectively.
3’-SL has been detected in bovine milk ranging from range from 47 to 55 mg/L and over 1 g/L in bovine colostrum (Albrecht et al., 2014; Aldredge et al., 2013; Urashima et al., 2013).
Human breast milk contains a family of structurally related oligosaccharides, known as human milk oligosaccharides (HMOs), as the third largest solid components (Bode, 2012; Kunz & Rudloff, 1993; Newburg, 2013). The concentrations of HMOs in human colostrum are 20 to 25 g/L, whereas in mature human milk, the concentrations are 5 to 20 g/L (Bode, 2012; Thurl et al., 2010; Urashima et al., 2018).
3’-SL belongs to the subfraction of ‘acidic’ HMOs, which is characterised by the presence of sialic acids, and the whole subfraction accounts for 1.5 – 3.3 g/L (Bode, 2012; Rijnierse et al., 2011; Thurl et al., 2010).
There are two naturally occurring sialyllactoses, which are constitutional isomers. 3’-SL is sialylated by α-(2"-3’) linkage and 6’-SL is sialylated by α-(2"-6’) linkage. 6’-SL is the predominant acidic HMO in human milk; however, both forms are reported to have similar functions and biological roles (Tarr et al., 2015).
Thurl et al. (2017) summarised the findings from twenty-one published studies and reported that the content of 3’-SL in milk from mothers who delivered at term ranged from 0.14 to 0.24 g/L (average 0.19 g/L). For mothers who delivered preterm, 3’-SL ranged from 0.21 to 0.36 g/L (average 0.29 g/L). More recently, Soyyılmaz et al. (2021) reported that a mean of mean concentrations of 0.19 g/L for mature milk, with a maximum mean of 0.70 g/L.
Using the reported levels of 3’-SL in human breast milk from Soyyılmaz et al. (2021) and considering the average and high daily intake of breast milk (800 mL and 1,200 mL, respectively) for infants from 0 to 6 months (EFSA NDA Panel, 2013), the daily intake levels of 3’-SL from human milk for a 6.7 kg body weight infant (EFSA, 2012) are shown in Table 4.
The history of use does not indicate any further areas for evaluation.
2.7. Proposed Use and Intake
The target population is the general population.
The intended uses and use levels of the 3’-SL sodium salt from the novel food are listed in Table 5. These food categories and intended use levels are the same as those assessed by EFSA for 3’-SL sodium salt produced by fermentation with a genetically modified strain of E. coli K-12 DH1 (EFSA NDA, 2020).
The anticipated intake for 3’-SL sodium salt in children up to the age of 16 weeks is estimated to be 52 mg/kg body weight/day for a 6.7 kg infant. This value was calculated from the use of 3’-SL in infant formula (0.2 g/L) at a high consumption level of 260 ml/kg body weight/day, as established by the EFSA Scientific Committee (EFSA SC, 2017). This value does not exceed the estimated high daily intake of 3’-SL in breast-fed infants per kg/BW (see Table 4).
An intake assessment using the summary statistics of consumption from the dietary surveys in the EFSA Comprehensive database was conducted by matching the intended conditions of use with the FoodEx2 categories. The estimated mean and high-level intakes of 3’-SL sodium salt from the proposed conditions of use for each sub-population are presented in Table 6.
The highest estimated 95th percentile intake for 3’-SL sodium salt from the novel food was reported in the infant sub-population at 71 mg/kg BW/day. By comparison, the highest estimated intake for 3’-SL from human breast milk consumption is 125 mg/kg BW/day in infants (Table 4).
The use level for 3’-SL sodium salt in food supplements is 0.2 g/day for infants, 0.15 g/day for young children, and 1.0 g/day for all other population sub-groups. Food supplements are not intended to be used if other foods with the novel food are consumed on the same day. For infants and young children, food supplements are not intended to be used if breast milk or other foods with added 3’-SL sodium salt are consumed on the same day.
On a body weight basis, the maximum intake of 3’-SL sodium salt in food supplements for infants (0.2 g/day) and young children (0.15 g/day) would be 30 mg/kg BW/day and 13 mg/kg BW/day, respectively. For all other sub-populations, the maximum daily intake would range from 14 to 43 mg/kg BW/day based on default body weight (EFSA, 2012). On this basis, consumption of 3’-SL sodium salt in food supplements is not expected to exceed the levels found in human breast milk.
3’-SL is already authorised for food categories other than those listed in Table 5 (assimilated Commission Implementing Regulation (EU) 2023/113). Therefore, an assessment of the combined intake of authorised and intended food categories was conducted. The estimated combined mean and 95th percentile intakes for each sub-population are presented in Table 7.
The highest combined estimated 95th percentile daily intake is reported in infants and young children at 75.9 and 77.3 mg/kg BW/day, respectively. These values are higher than the estimated daily intake from the intended uses alone (Table 7), but below the high estimate for 3’-SL daily intake from human milk of 125 mg/kg BW/day (Table 4).
2.8. Absorption, Distribution, Metabolism and Excretion (ADME)
No ADME studies were conducted on the novel food.
3’-SL does not undergo any significant digestion by human enzymes in the upper gastrointestinal tract and only small amounts are expected to be absorbed (EFSA NDA Panel, 2020). HMOs are fermented in the colon by intestinal microbiota with a fraction excreted unchanged in the faeces and a small fraction found in the urine (EFSA NDA Panel, 2022). There is no information which indicates that 3’-SL in the novel food differs from the 3’-SL in human breast milk (EFSA NDA Panel, 2023).
The ADME of human milk oligosaccharides are well understood and the information does not indicate any further areas of concern.
2.9. Nutritional Information
The novel food is mainly composed of the oligosaccharide, 3’-SL as a sodium salt, which is structurally identical to the naturally occurring counterpart in human breast milk.
The novel food contains 3’-SL sodium salt which may contribute to the daily intake of sodium in consumers. The EFSA NDA Panel has provided advice on the levels of sodium that are considered adequate and safe (FSA NDA Panel, 2019).
The estimated highest intakes of sodium from 3’-SL sodium salt consumption are shown in Table 8.
Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10. Toxicological Information
Toxicological studies were performed with 3’-SL sodium salt to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as proprietary data. They were reviewed by the ACNFP and considered essential in the assessment of the safety of the novel food.
2.10.1. Genotoxicity
In vitro genotoxicity testing of 3’-SL sodium salt was conducted under Good Laboratory Practice (GLP) conditions and according to the OECD guidelines: in vitro bacterial reverse mutation test (OECD TG 471) and in vivo mammalian erythrocyte micronucleus test (OECD TG 474). This is not the approach recommended by the UK Committee on Mutagenicity or in the guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283.
The Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA, 2011) recommends two in vitro tests as the first step in testing for genotoxicity: in vitro bacterial reverse mutation test (OECD TG 471) and in vitro mammalian cell micronucleus test (OECD TG 487). This approach addresses the key endpoints for adequately assessing genotoxicity with the minimum number of tests and avoiding unnecessary animal tests.
The in vivo mammalian erythrocyte micronucleus test (OECD TG 474) assesses both structural and numerical chromosomal aberrations and is an appropriate follow-up test for in vitro clastogens and aneugens.
The in vitro bacterial reverse mutation test (Oguma, 2019 [unpublished]) demonstrated that 3’-SL sodium salt is non-mutagenic at concentrations up to 5,000 µg 3’-SL/plate, in the absence or presence of metabolic activation.
The in vivo mammalian micronucleus test (Kikuchi, 2020 [unpublished]) reported that that 3’-SL sodium salt is non-clastogenic and non-aneugenic. However, no evidence was provided to demonstrate that the bone marrow in mice had been exposed to 3’-SL sodium salt. Therefore, an in vitro micronucleus test was conducted.
The in vitro micronucleus test (Kikuchi, 2022 [unpublished]) demonstrated that 3’-SL sodium salt is non-clastogenic and non-aneugenic in the absence or presence of metabolic activation up to the highest concentration of 2,000 µg 3’-SL/ml.
The results from these in vitro and in vivo studies support the conclusion that 3’-SL sodium salt is not genotoxic.
2.10.2. Sub-chronic toxicity
A Repeated Dose 90-day oral gavage study in rodents (Tsuboi, 2021 [unpublished]) was conducted under GLP conditions according to OECD TG 408 guidelines as recommended by the Guidance on the preparation and submission of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283. The aim of the study was to identify any adverse effects following the consumption of the novel food.
In this 90-day oral gavage study, each group consisted of 10 female and 10 male Sprague Dawley rats which were dosed with 0 (control – vehicle only [water]), 502, 1,003 or 2,007 mg/kg BW/day of 3’-SL sodium salt by oral gavage.
No deaths, test item-related clinical abnormalities, ocular changes, or differences in bodyweight between test groups were reported. Statistically significant changes in food consumption, haematology, clinical chemistry, urinalysis, and organ weights were reported. However, these were not dose dependent and the applicant has confirmed these were within the historical control range for the facility and therefore not of concern.
No dose related abnormalities were noted during the necropsy or histopathological evaluation. Therefore, the no observable adverse effect level (NOAEL) for 3’-SL sodium salt was considered to be the highest dose tested of 2,007 mg/kg BW/day.
2.10.3. Human studies
No human clinical trials were conducted with the novel food.
2.11. Allergenicity
The protein content of the novel food is reported as < 0.01% w/w.
Absence of bacteria from the Enterobacteriaceae family (ISO 21528-1:2017) confirmed that the genetically modified E. coli NEO3 is not present in the novel food.
The potential allergenicity of the introduced proteins expressed in E. coli W was assessed using the National Institute of Health Sciences (Japan) Allergen Database for Food Safety and conducted in line with FAO (2001) guidelines. None of the proteins was predicted to be an allergen.
Two ELISA (enzyme linked immunosorbent assay) tests were conducted on the novel food manufactured using soy peptone to detect the presence of milk proteins (LOQ = 1 µg/g). The results confirmed that milk proteins were effectively removed during the purification process and were not present in the finished powder.
Five batches of the novel food manufactured using soy peptone were also tested for the presence of soybean protein. The results from the ELISA test (LOQ = 1 µg/g) confirmed that soybean protein was effectively removed from all batches of the novel food during the purification process and was not present in the finished powder.
The likelihood of allergenic reactions to the novel food is expected to be low under the proposed conditions of use.
3. Discussion
The novel food is a white to off-white powder which is mainly composed of the human identical milk trisaccharide, 3’-SL as a sodium salt (≥ 82.0% w/w DM), as well as other saccharides in smaller quantities.
The novel food is manufactured by microbial fermentation using a genetically modified strain of Escherichia coli W and then refined to yield the purified powder.
3’-SL sodium salt is intended to be used in dairy products and analogues, bakery wares, foods for special groups, beverages, and food supplements. The general population are identified as the target population of the novel food.
Analysis confirms that the 3’-SL sodium salt is structurally identical to the 3’-SL found naturally in human milk. Exposure to 3’-SL relates solely to breastfeeding infants as there is no recognised history of use for this milk oligosaccharide as an ingredient in foods or food supplements.
In the Repeated Dose 90-day oral gavage study in rodents, the NOAEL for 3’-SL sodium salt was 2,007 mg/kg BW/day, the highest dose tested. When this NOAEL is compared with the highest estimated exposure in each population category, the margins of exposure range from 28 to 502. Given that the 3’-SL sodium salt in the novel food is equivalent to 3’-SL found in human breast milk, these margins of exposure are acceptable with respect to the highest estimated daily intakes in the intended population.
The anticipated daily intake of 3’-SL sodium salt in all population groups, including children up to the age of 16 weeks using infant formula alone, is not expected to exceed the highest intake level of 3’-SL in breastfed infants on a body weight basis.
The use level of 3’-SL sodium salt in food supplements (0.2 g/day for infants, 0.15 g/day for young children and 1.0 g/day for all other sub-populations) is not expected to exceed the highest intake level of 3’-SL in breastfed infants on a body weight basis. Food supplements are not intended to be used by the general population if other foods containing the novel food, including breast milk or other foods for infants and young children, are consumed on the same day.
4. Conclusions
The FSA and FSS have undertaken the assessment of the novel food, which is composed mainly of 3’-SL sodium salt and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake level and the proposed use in food and food supplements was not considered to be nutritionally disadvantageous.
The advice was based on the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
-
annexes to the dossier which relate to the identity of the novel food, the production process, composition, stability, and the solubility of the novel food
-
in vitro bacterial reverse mutation test (Oguma, 2019 [unpublished]); in vitro micronucleus test (Kikuchi, 2022 [unpublished]); 90-day repeat dose oral gavage study with the novel food (Tsuboi, 2021 [unpublished])
Abbreviations
Acknowledgements
The members of the ACNFP during the course of the assessment who were;
Dr Camilla Alexander White, Dr Anton Alldrick, Dr Kimon Andreas Karatzas, Ms Alison Austin, Professor George Bassel, Dr Mark Berry, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Catharina Edwards, Professor Susan Fairweather-Tait, Professor Paul Frazer, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Mrs Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.
Article updated on 18th October 2024