Executive Summary
There has been increasing attention on the role that insects can have in the production of protein for inclusion in animal feed, whilst also reducing the volume of organic waste streams. Currently in the UK and the EU the material that can be used as a rearing substrate for insects for production of protein for feed and food is regulated and waste streams that contain or may potentially contain animal by-products (ABPs) are not permitted to be used to rear the insects. The aim of this study was to provide chemical and microbiological data from model insect rearing systems using four currently non-permitted rearing substrates as a basis to assess the potential risk from use of these materials.
Selection of the materials to be tested was based on the results of a questionnaire to key stakeholders with interest in insect bioconversion and discussion with the FSA. The materials selected for testing were:
-
Supermarket surplus containing animal by-products (ABPs) (Supermarket)
-
Food processing surplus containing ABPs (Manufacturing)
-
Kitchen waste from hospitality sector containing ABPs (Catering)
-
Broiler poultry manure (Poultry manure)
These materials were used to rear black soldier fly (BSF) larvae and samples of the rearing substrate, the larvae and the frass were taken for analysis of chemical and microbiological contaminants. Samples obtained from a UK insect producer using currently permitted rearing substrates were also included.
Analytical methods screened for 745 chemical analytes (metals, veterinary medicines, pesticides, mycotoxins, polycyclic aromatic hydrocarbons (PAHs) (kitchen waste only), nitrate/nitrite, perfluoroalkyl and polyfluoroalkyl substances (PFAS)), the presence of key microbial organisms was assessed, and non-targeted screens were used to assess the presence of natural toxins and viral RNA that were present in the samples.
Of the chemical analytes screened for, a total of 101 were found in the larvae. The majority of these were metals (58). Of the analytes found, there were no exceedances for any of the chemicals where maximum limits are specified in feed materials of animal origin. However, some pesticide residues in larvae reared on supermarket surplus and poultry manure exceeded the MRLs for terrestrial invertebrates which may have implications if the larvae were to be used as food.
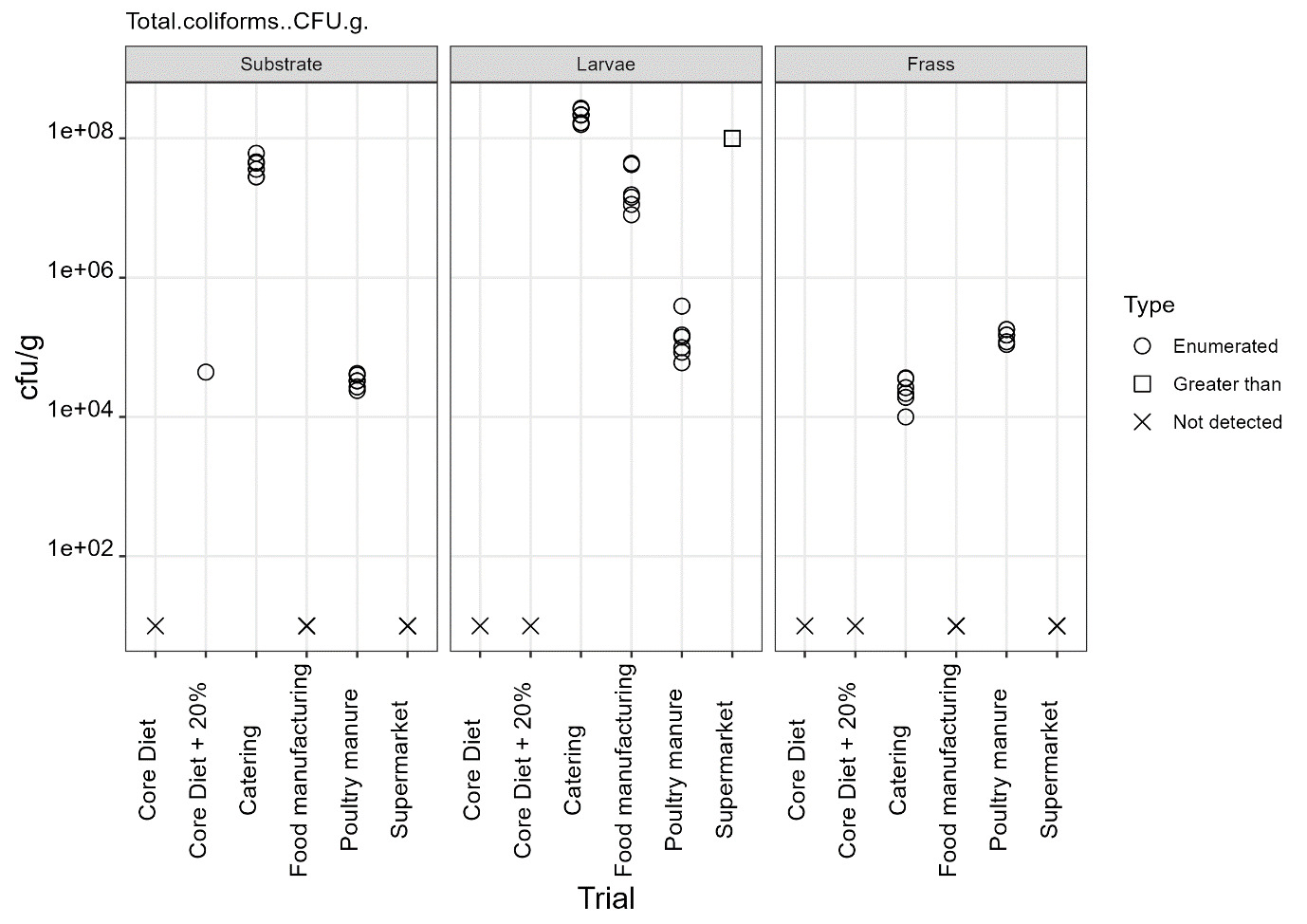
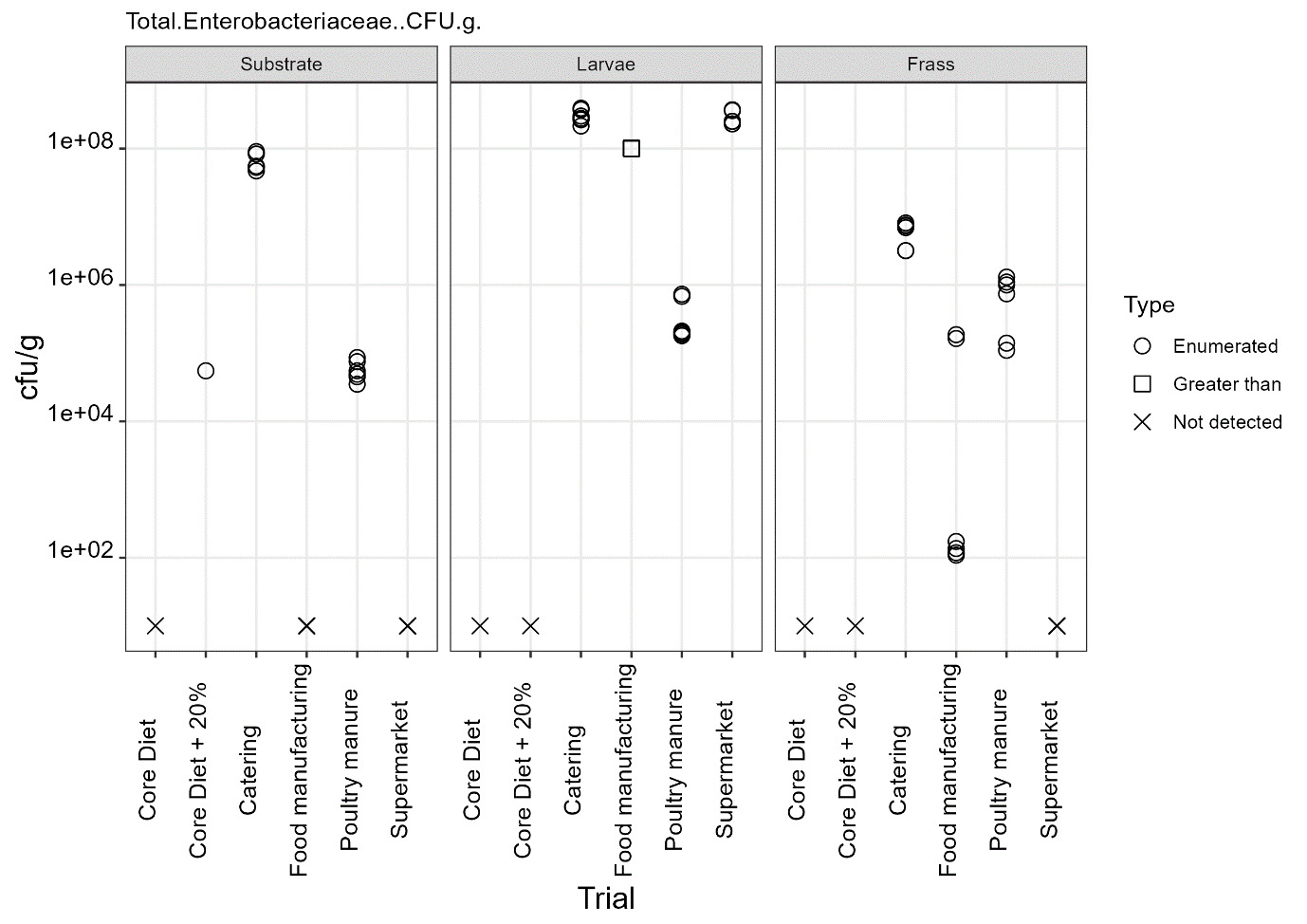
The only regulatory limit exceeded for feed ingredients of animal origin was for the presence of Enterobacteriaceae in larvae reared on all four of the currently non-permitted substrates. The regulatory limit for Enterobacteriaceae in feed materials of animal origin is 300 cfu/g. However, in this study for the currently non-permitted substrates only minimal processing was used to kill the larvae. The regulatory levels specified refer to samples taken after the application of a processing method. The larvae reared on the baseline samples complied with the regulatory limits for Enterobacteriaceae for feed ingredients of animal origin. These larvae were culled by blanching in boiling water (approx. 100°C) and cooking until core temperature exceeded 75°C according to the producer’s protocols.
The results from this study illustrate that further processing of larvae is required to reduce the microbial load. It is likely that processing methods typically used by industry for the production of insect protein would significantly reduce the level of these organisms, as demonstrated by the results from the baseline samples, which were supplied by a UK insect producer using their standard protocols. However, this would need to be confirmed and supported by HACCP and GMP procedures.
There was evidence of bioaccumulation in the larvae for some compounds. Cadmium was shown to bioaccumulate in BSF larvae as previously reported. There was also evidence of bioaccumulation of other metals including magnesium, calcium, and phosphorus in larvae reared on both currently permitted and currently non-permitted substrates. Although this may not result in a direct safety concern, this may have implications for some metals that serve as macro- or micronutrients in animal feeds.
There was also evidence of bioaccumulation of didecyldimethylammonium chloride (DDAC) and haloxyfop in larvae reared on poultry manure. Other contaminants such as mycotoxins and PAHs also gave indication for potential bioaccumulation but variation between samples means that further testing would be needed to ascertain whether this is the case.
As far as the authors are aware this is the first study that has examined the presence of PFAS through the insect bioconversion process using naturally occurring levels of these compounds. Some PFAS were found in the larvae and the frass, but a more extensive study is recommended to confirm these findings.
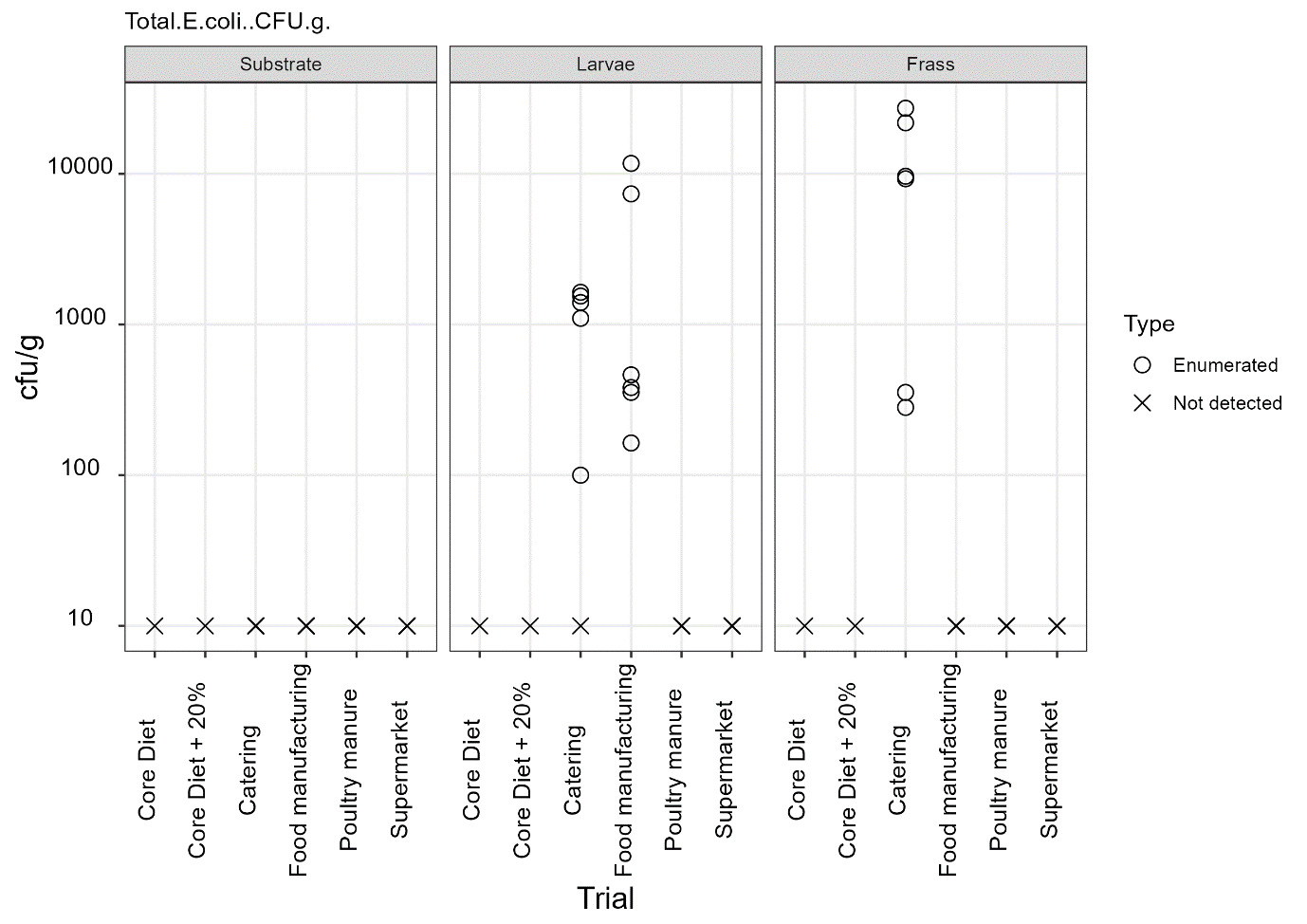
Commission Regulation (EU) No 142/2011 specifies that processed manure taken during or immediately after processing should comply with limits Salmonella spp. and Escherichia coli or Enterococcaceae. The maximum value of 1000 cfu/g was exceeded for Enterococcaceae in frass samples from all rearing substrates but was only exceeded for E. coli in frass from catering waste. These results indicate that further processing of the frass from currently permitted and non-permitted substrates is required to conform to regulatory requirements.
The non-targeted viral screen confirmed the presence of RNA from plant and animal pathogens in larvae and frass, but the infectivity of these viruses could not be elucidated from this study. Infection studies should be carried out to determine if this is a risk, particularly for plant pathogens when frass is used as a fertiliser.
The non-targeted toxin screen demonstrated the presence of natural toxins such as solanidine. Currently there are no regulatory limits for the presence in animal feed and an EFSA risk assessment concluded that a risk characterisation of potato glycoalkaloids in feed for farm and companion animals was not possible due to insufficient data on potential adverse effects in these species (EFSA, 2020). Data on the presence of these compounds in a range of animal feeds is therefore required before an assessment of the levels found in insect larvae can be evaluated.
It was noted that several compounds were not detected in the rearing substrate but were detected in the larvae and/or the frass. It is considered that this may be due to the greater homogeneity of the larvae and the frass samples compared with the rearing substrate such that there may have been a more even distribution of any contaminant present.
Examination of the variation in results between replicates showed that between replicate variation occurred mainly for the rearing substrate. This is likely to be due to the rearing substrate being less homogenous than the larvae and the frass. It is therefore recommended that when assessing contaminants in rearing substrate a greater number of samples are assessed.
Finally, it is important to note that only a single source from each category was evaluated in this study and the samples obtained were taken over a short time period. More extensive testing across a wider range of suppliers and to reflect potential changes in seasonality is recommended.
Introduction
There has been increasing attention on the role that insects can have in the production of protein for inclusion in animal feed, whilst also reducing the volume of organic waste streams. The focus for insect-derived protein for inclusion in animal feed has predominantly been on use of the black soldier fly (BSF; Hermetia illucens) and the yellow mealworm (Tenebrio molitor). Studies in the field of insect bioconversion have expanded over the past two decades leading to the funding and build of commercial scale operations in North and South America, Europe, Southeast Asia and Australia.
In many countries materials to be used in animal feedstuffs are subject to regulations to ensure safety for the animals and human consumers. In the EU and the UK there are regulations that define the species of insect that can be used to produce insect protein for feed and food, the waste streams that the insects can be fed, and the animals that the resultant insect protein can be fed to.
Insects reared for inclusion in animal feed are defined in the EU Animal By-Products’ legislation (Article 3(6) of Regulation (EC) No 1069/2009) as farmed animals. Only feed ingredients that can be used for feeding of other farmed animals can be fed to insects. This restricts the rearing substrates for the insects to vegetal based materials. Animal derived products, with a few exceptions, are not currently permitted. Exceptions to the use of animal by-products (ABPs) as insect rearing substrates include dairy products (e.g., milk (if pasteurised), cheese and eggs (if cooked), fishmeal, gelatine, collagen, hydrolysed proteins and blood products (non-ruminant).
Although vegetal based substrates such as brewery by-products and fresh fruit and vegetables are a good rearing substrate for certain insect species, species such as the BSF are able to develop on a wide range of organic substrates including those containing meat and fish and animal manures. The substrate on which the larvae are reared affects many aspects of the insect bioconversion process in addition to the nutritional composition of the larvae. For example, the resultant frass will have different properties depending on the waste source (Klammsteiner et al., 2020). It has also been shown that the life cycle assessment for the production of insect protein is dependent on the rearing substrate and it has been concluded that the use of food processing by-products, wastes or manures may be needed to reduce the environmental impacts of the insect bioconversion process (Smetana et al., 2021).
Potential contaminants (chemical and microbiological) and the associated risk will also be related to the material used as a rearing substrate and could affect the safety for use as an animal feed ingredient of the resultant larvae and the use of the frass.
Maximum limits for certain chemicals in animal feed are provided by the EU Directive on Undesirable Substances and Products (Directive 2002/32/EC) and the EU Regulation on Pesticides Maximum Residue Levels (MRLs) (Regulation (EC) No 396/2005). There are also limits specified in (Regulation (EC) 142/2011) for the presence of Salmonella and Enterobacteriaceae in feed materials of animal origin.
The safety of insect derived products with regard to chemical contaminants has been recently reviewed by Meyer et al. (2021). The review concluded that the heavy metals cadmium, mercury, lead and arsenic can bioaccumulate in insects, whilst mycotoxins and PAHs do not. A lack of data for PAHs, plant toxins, dioxins and PCBs was also highlighted. Vandeweyer et al. (2021) reviewed safety with regard to biological contaminants and it was concluded that data on prions, foodborne viruses and foodborne parasites is still needed and their fate during insect processing requires investigation. The presence of microorganisms and the need to assess these in production areas in addition to present in the rearing substrate was also highlighted (Vandeweyer et al., 2021). More recent studies have also examined physical contaminants such as microplastics (Lievens et al., 2023).
There are several studies that have shown that some insect species will bioaccumulate certain heavy metals. The degree of bioaccumulation depends on the insect species and the metal. For example, BSF will bioaccumulate cadmium (Charlton et al., 2015; Gao et al., 2017; Purschke et al., 2017), whilst yellow mealworm larvae have been reported to bioaccumulate arsenic (van der Fels-Klerx et al., 2016).
The uptake of mycotoxins has also been studied although studies have tended to focus on specific mycotoxins, mainly aflatoxin B1, zearalenone, deoxynivalenol, ochratoxin A and fumonisin B1 and B2 (Meyer et al., 2021 and references therein) and research looking at a wider range of potential mycotoxins is limited. Generally, spiked materials have been used in these studies and it has been concluded that BSF larvae do not bioaccumulate mycotoxins from the rearing substrate (Bosch et al., 2017; Camenzuli et al., 2018; Purschke et al., 2017). The levels of mycotoxins found in larvae in these studies were very low or below the limit of quantification. Metabolism of certain mycotoxins has been demonstrated in T. molitor larvae (Camenzuli et al., 2018; Purschke et al., 2017). However, in some studies examining the mass balance, an inability to account for all the material, either as the native mycotoxin or known metabolites, led to the conclusion that further research on the metabolism of mycotoxins by different insect species is required (Camenzuli et al., 2018).
There is limited research on uptake of pesticides, veterinary medicines, PAHs (Meyer et al., 2021) and of other contaminants such as microplastics, but data is lacking on the risk from different rearing substrate types. For example, some contaminants such as veterinary medicine residues may be more likely to be found in materials containing animal derived products than in those of plant origin.
Ffoulkes et al. (2021) examined 22 potential substrates that could be used for rearing insects such as BSF and concluded that at least ten by-product streams could be used by the UK insect industry. Some of these such as brewers’ grains and vegetable by-products are currently permitted for use and are already used by insect producers. Other waste streams identified are not currently permitted as they contain or may potentially contain ABPs. The waste streams identified were further divided by the authors into two categories; achievable and aspirational based on consideration of legal, social, and practical challenges (Ffoulkes et al., 2021).
The aim of this study was to provide chemical and microbiological data from model insect rearing systems using four currently non-permitted rearing substrates. Analytical data from the study provides a key data set to be used in subsequent risk assessments.
Key objectives of the study were:
-
To obtain representative material from the chosen streams and to rear the chosen insect species on the selected streams.
-
To compare with baseline samples provided by a commercial insect supplier that have been reared on a permitted substrate.
-
To conduct comprehensive chemical and microbiological analysis of the rearing substrate, larvae, and the frass.
-
To complete a literature review on the potential allergen presence and subsequent risk that the allergens could be carried through the process from the substrate to the larvae and subsequently to the farmed animal.
1. Selection of substrates for testing
The current project allowed for the assessment of four rearing substrates. To aid in the selection of the four substrates an online questionnaire was used to seek the opinions of key stakeholders, including insect producers, insect protein users and academics, with interests in insect bioconversion. Opinions were sought for ten categories, which were based on the achievable and aspirational categories identified by Ffoulkes et al. (2021). The ten categories were:
-
Mixed food surplus from retail containing ABPs
-
Mixed food manufacturing surplus containing ABPs
-
Mixed food surplus from hospitality and food service containing ABPs
-
Domestic food surplus containing ABPs
-
Poultry manure – Layers
-
Poultry manure – Broilers
-
Pig manure
-
Cattle manure
-
Anaerobic digestate – Food-based
-
Anaerobic digestate – Manure-based
For each of these categories the following questions were posed with the selections for responses, if provided, also shown:
- If approved, would your business consider using this substrate to rear insects or including insects reared on it into your supply chain?
Options for response: Yes, No, Maybe
-
Do you have any concerns for the use of this substrate from the point of view of:
-
Market acceptance
-
Substrate availability
-
Safety
Options for response: Yes, No
-
If you answered yes, please give your reasons.
-
Are there any specific examples of this substrate type that you would like to see tested in this category?
Additional questions not confined to the potential substrate categories were:
-
Are there any substrate types, which haven’t been mentioned, that you would ideally like to be examined?
-
Do you have any further thoughts or considerations on this project that you would like to share with us?
The questionnaire was sent to eleven stakeholders, either individuals or representatives of larger interested bodies, representing insect production companies, industry lobbying groups, the broader farming community, retailers and academics.
Following compilation of the responses the data was reviewed by project stakeholders and the decision on the four substrate categories to be included in the study was made.
Results and observations
All 11 consultees responded with completed questionnaires. Whilst it is noted that 11 responses are a low number, all responses were from individuals, companies or member groups with knowledge of insect bioconversion and the current opportunities and challenges in the UK.
The main observations from the responses are summarised below by the source category.
Food based categories
Respondents indicated that they would consider the use of the food surplus categories as a substrate to rear insects or inclusion of insects reared on these substrates in their supply chain. The exception to this was domestic food surplus where 55% of respondents indicated that they would not use this or were undecided (Figure 1A).
For all four categories substrate availability was generally not considered to be of concern. Differences in the responses to concerns from the point of view of market acceptance and safety were observed with respondents indicating concerns for using domestic food surplus, particularly for safety (73% of respondents; Figure 1B).
Manure-based categories
There was a noticeable difference in the number of respondents indicating that they would consider the use of the manure-based categories as a substrate to rear insects or inclusion of insects reared on these substrates in their supply chain depending on the source of the manure. A greater number indicated that they would consider use of poultry manure from either broilers or layers (64% and 55% respectively) than for using pig manure (36%) or cattle manure (18%) (Figure 2A).
For all four manure types, availability was not considered a concern, but safety and market acceptance were of concern (Figure 2B).
Comments indicated that the risk from microbial contaminants such as Salmonella was a concern for this category (Appendix A). However, there was interest in testing this category as it was considered that this could be a solution for more problematic waste streams, but that uses for the larvae other than in the food and feed chain may need to be considered (Appendix I).
Anaerobic digestate-based categories
There was a noticeable difference in the number of respondents indicating that they would consider the use of the anaerobic digestate-based categories as a substrate to rear insects or inclusion of insects reared on these substrates in their supply chain depending on the source of the anaerobic digestate. This difference reflected the differences previously seen with the food-based and manure-based categories with 64% of respondents indicating that they would consider use of food based anaerobic digestate and only 27% indicating that they would consider use of manure based anaerobic digestate (Figure 3A).
Availability of both sources of anaerobic digestate was not considered a concern but there were concerns on safety and market acceptance and a greater number of respondents had concerns for the manure-based anaerobic digestate (Figure 3B).
Additional comments from respondents are shown in Appendix A.
Based on the responses and following review and discussion with FSA risk assessment and policy staff, four categories were selected for testing in this project. These were:
-
Supermarket surplus containing ABPs (Supermarket)
-
Food processing surplus containing ABPs (Manufacturing)
-
Kitchen waste from hospitality sector containing ABPs (Catering)
-
Broiler poultry manure (Poultry manure)
2. Insect rearing trials
2.1. Sample supply and preparation
Currently permitted rearing substrates
Baseline samples of rearing substrate, larvae and frass were provided by Better Origin. Two types of rearing substrate designated as Core Diet and Core Diet +20% were used. The rearing substrates were comprised of:
-
Core Diet - fruit & vegetables (apples, potatoes, tomatoes) and bakery waste (bread loaves)
-
Core Diet +20% – As for Core Diet with the inclusion of 20% grain and grain by-products (wheat bran, spent brewers grains)
Feedstocks were shredded using a mechanical shredder (Voran), to <5 mm particle size, and blended. The feedstocks were inoculated with silage additive (Provita Advance Plus applied in accordance with the manufacturer’s recommendation), to ensure lactofermentation. pH was monitored throughout use of the feedstock.
Sampling of currently non-permitted rearing substrates
Sampling was undertaken to ensure that the samples of rearing substrate were as representative as possible. Representative means that the average proportion of components and concentration of contaminants in the sample is close to the true equivalent values in the material that sample was taken from, that is, the material that the sample represents.
There is a broader kind of representativeness: the extent to which the specific materials we sampled were themselves representative of the broader population of that kind of material: for example, the extent to which the contaminants in the supermarket waste that we took samples were representative of supermarket waste from other supermarkets or at other times of year. While we took efforts to ensure that the samples we took were representative of the materials from which they were taken, the extent to which those materials were representative of the broader population was outside of the scope of this project and remains an unknown.
For the collection of all trial rearing substrates, visits were made to the suppliers to find out how and where the materials were produced and stored. Discussions were held with staff about the best way to collect the material to ensure it was as representative as possible. With the exception of the broiler poultry manure, the material for testing was collected on two separate occasions, to allow for variation over time.
The rearing substrates were homogenised prior to use as described below. Equal quantities of the rearing substrate at each feeding point were combined and samples taken to provide two bulk samples (A and B). One of these bulk samples was analysed once (A) and the second bulk sample was analysed twice (B1 and B2; Figure 4). Differences between the concentration of contaminants in A and B samples were assessed to give an indication of the representativeness of the sampling.
Currently non-permitted rearing substrates
Manufacturing Surplus
Two 30 kg batches of manufacturing surplus containing animal-by-products were delivered to Fera from a local food manufacturer on trial day -2 and 5. Both batches consisted of 10 kg raw puff pastry, 10 kg cooked pastry products (primarily pork sausage rolls), and 10 kg of raw beef trimmings (primarily sinew and cartilaginous tissue) (Figure 5). This was distributed into two plastic containers, sealed, and left at ambient temperature (approximately 20°C) for 48 h to simulate a collection and distribution period. The material was then homogenised into a paste and transferred to a cold store (1-4oC) for use during the trial.
Supermarket surplus
Two 30 kg batches of supermarket surplus containing animal-by-products were transferred to Fera from a local supermarket on trial day -2 and 5. This consisted of assorted items that were past the use by or best before date and were set aside for collection by the supplier. The waste consisted of approx. 53% bread, 8% deli meats (e.g., ham and charcuterie), 8% coleslaw, 7% raw beef, 6% raw and cooked chicken products, 5% cheese, 3% olives and deli counter veg (e.g., pickles and sundried tomatoes), 3% raw pork, 3% ready meals (e.g., spaghetti bolognaise). 2% desserts (e.g., yoghurt and tiramisu) (w/v). This was distributed evenly into two containers, sealed, and left at ambient temperature (approximately 20°C) for 48 h to simulate a collection and distribution period. The material was then homogenised and transferred to a cold store (1-4°C) for use during the trial.
Catering Waste
Two 30 kg batches of pre-consumer kitchen waste containing animal-by-products collected over approximately 24 h were transferred to Fera from a local restaurant on trial day -2 and 5. The waste consisted of assorted items classed as past the use by date or defined as unsuitable for retail within the restaurant. This waste consisted of approximately 50% cooked chicken, 10% coleslaw, 10% partially cooked chipped potatoes, 10% white bread rolls, and 20% (w/v) of other unidentified food items or ingredients typically found on the restaurant’s menu such as sweetcorn, salad leaves and cheese (primarily halloumi) (Figure 7). This was distributed into two plastic containers, sealed, and left at ambient temperature (approximately 20°C) for 48 h to simulate a collection and distribution period. The food was then homogenised and transferred to a cold store (1-4°C) for use during the trial.
Poultry Manure
Broiler poultry manure (40 kg) from a shed at the end of a production cycle was collected from a local farm. Manure was selected from random locations in the poultry shed by Fera staff and transferred to site on trial day -5. The waste consisted of chicken manure, un-eaten feed, wood shavings, and sawdust. The manure required hydration to make it suitable for development of the larvae and this was achieved by adding 100 ml of water for every 70 g of substrate. The hydrated manure was stored in a cold store (1-4°C) and left to hydrate for 48 hours before the trial commenced.
2.2. Production of BSF larvae and frass
Production of BSF larvae and frass on currently permitted rearing substrates.
BSF larvae were reared by Better Origin from hatch to 5 days old (mass of a single larva approx.10 mg) on a seedstock comprising of an enriched Gainesville diet. Larvae were then transferred to 600 x 400 mm rearing trays containing Core Diet or Core Diet + 20%. Larvae were fed daily following a predetermined feed curve. Larvae were maintained at 28°C, 55-60% relative humidity (r.h.).
After 12 days of rearing, larvae were harvested through mechanical sieving. The larvae were washed with water to remove residual rearing substrate on the surface and were then culled by blanching in boiling water (approx. 100°C) and cooking until core temperature exceeded 75°C. The exact time will depend on the quantity of larvae and the size of the vessel The larvae were then drained, cooled at room temperature, bagged and frozen.
Data from the rearing of the larvae on the substrates provided was not available, but typically the biomass conversion ratio for the core diet is 6.6 and for the core diet +20% is 4.5 with average yield per tray of 1.1 kg and 1.7 kg for the core diet and core diet + 20% respectively.
Samples of the two rearing substrates and the larvae reared on these together with the resulting frass were sent to Fera Science Ltd. Samples were supplied frozen and were stored in a freezer at approximately -18°C prior to analysis.
Production of BSF larvae and frass on currently non-permitted wastes
Neonate larvae approximately 0-24 h old were used in all studies. Each trial was carried out using a nursery container for days 0 to 7, then transferring the larvae to a rearing tray system from day 7 to trial termination.
For each replicate the rearing substrate (500 g) was weighed into a 2.7 litre rearing container (nursery container) and neonate larvae (250 mg) were added. Lids with five 8 mm diameter holes were put on the containers and the containers were placed into a rearing room at 27°C, 70% rh. After 7 days the contents of the nursery container were transferred to a 600 mm by 400 mm rearing tray. Larvae were monitored and re-fed at set periods. Prior to each feed 50 larvae were removed, washed, and dried, and weighed to monitor growth. There were six replicate trays for each rearing substrate tested.
Trials were terminated when the first pre-pupae were identified, with larvae then separated from the frass. Total larvae mass and mass of remaining frass/feed substrate were determined.
Dry matter readings of the rearing substrate were determined before trial set up. Some substrates had moisture content adjusted to provide approximately 30% dry matter content or to improve the consistency of the substrate for the larvae to consume, whichever was deemed most appropriate for success of the trial. Due to the variance in nutritional quality between each substrate, adaptations were made to each trial and are highlighted below.
Trial 1 – Manufacturing surplus
Dry matter content was determined and was adjusted to approximately 45% dry matter prior to feeding. The first batch of manufacturing surplus was used for feed on trial day 0; the second batch was used for feeding on days 7 and 12. The trial ran for 14 days.
Trial 2 – Supermarket surplus
Dry matter content was adjusted to approximately 30% prior to feeding. The first batch of supermarket surplus was used for feed on trial day 0; the second batch was used for feeding on days 9 and 13. The trial ran for 15 days.
Trial 3 – Catering Waste
Dry matter content was adjusted to approximately 32% dry matter prior to feeding. The first batch of catering waste was used for feed on trial day 0; the second batch was used for feeding on days 7 and 10. The trial ran for 14 days.
Trial 4 – Poultry Manure
Dry matter content was adjusted to approximately 22% dry matter prior to feeding. The BSFL were fed on days 0, 7, and 10. Adaptations to the protocol were made to enable feeding and data collection to be performed in a Class II microbiological safety cabinet. Adaptations included starting the trial in twelve nursery containers with 125 mg of neonate larvae, then combining two nursery containers randomly on day 7 when transferring to a 600 mm by 400 mm rearing tray. The trial ran for 15 days.
Sampling
On trial termination samples were collected for use in safety testing. The objective of the sampling method was to obtain material that was representative of the whole bulk from which it was taken.
For sampling of larvae, twelve random handfuls were selected from each replicate and combined into a bulk sample. This was repeated to create a second bulk sample. A sub sample was taken from these bulks and sent for analysis. This was repeated for frass and the two bulk samples of feed. Samples for analysis of chemical contaminants were stored in a freezer for up to four weeks prior to analysis. For analysis, bulk A was analysed once and bulk B (B1+B2) was analysed twice (Figure 4).
Data analysis
The following parameters were calculated for each treatment:
Substrate reduction as a percentage was calculated on a wet weight basis as:
Substrate reduction (%) = (feed added - residue) ÷ feed added × 100
Biomass conversion ratio was calculated on a wet weight basis as:
Biomass conversion ratio = feed added ÷ larval mass
Bioconversion as a percentage was calculated on a wet weight basis as:
Bioconversion (%) = larval mass ÷ feed added × 100
Results
A sufficient quantity of larvae and frass for safety testing was obtained from all four rearing substrates. As expected, survival and mass of the BSF larvae was determined by the rearing substrate. Although the trials were set up to ensure development of the larvae, the objective for this study was to examine the safety of the larvae produced, and therefore optimisation of larval production was not examined in this project. Therefore, although general observations can be made regarding the different rearing substrates in this study it must be borne in mind that production of larvae can be improved for all substrates and comparisons between substrate types should be undertaken with caution. It must also be recognised that only a single source of rearing substrate was tested under these general headings and therefore the nature of the rearing substrate could change significantly between providers and throughout the year.
For some rearing substrate types e.g., food manufacturing and poultry manure, separation of the larvae from the frass at the end of the trial was difficult and although as many larvae as possible were separated the proportion of larvae remaining in the substrate may have been higher for some substrates than others leading to an underestimate of survival and the mass of larvae obtained.
In these trials, food manufacturing surplus provided the lowest mass of larvae and the highest biomass conversion ratio (Table 1). The nature of the food manufacturing surplus tested was very fatty and this will have impacted the development of the larvae. The larvae developed best on the supermarket surplus, where the highest mass of larvae and the lowest biomass bioconversion ratio was observed (Table 1).
There was some variation in the development of the larvae between replicates and for food manufacturing and supermarket surplus one replicate failed to produce larvae. The reason for this is not known.
Waste reduction was high (49.4% – 91.0% w/w) for all rearing substrates except poultry manure (29.7%), where it is possible that a higher proportion of uneaten substrate remained.
3. Safety testing
Materials and methods
Sample pre-processing / preparation
Core diets
Sub-samples for microbiological analyses were taken aseptically, after defrosting of the samples (rearing substrate, larvae, frass) from representative core diet and core diet+ 20% trials, to ensure they were representative of the original samples. The remainder of each sample was frozen before freeze-drying further sub-samples for a minimum of 48 h. After drying the samples were homogenised and aliquoted into sub-samples for the chemical analyses.
Sample pre-processing / preparation
Test diets
Sub-samples for microbiological analyses were taken aseptically for the three sample types (rearing substrate, larvae, frass). The remainder of each sample was frozen before freeze-drying further sub-samples for a minimum of 48 h. After drying the samples were homogenised and aliquoted into sub-samples for the chemical analyses.
Safety analysis
Metals
Deionized (18.2 MΩ cm) water, metal analysis grade reagents and acid cleaned plasticware were used throughout. Aliquots of sample were weighed into allotted digestion vessels and a mixture (4:1) of nitric acid and hydrochloric acid added. The vessels were capped, and the contents digested under high temperature and pressure using a single reaction chamber microwave digester system (Ultrawave, Milestone). Reagent blanks, certified reference materials and a spiked sample were also used in the procedure. The resulting solutions were transferred to pre-marked acid-cleaned plastic test tubes and diluted with deionised water. The digest solutions, together with a set of standards covering the expected concentration range, were internally standardised with indium in dilute nitric acid (1% v/v). Multi-element measurements were made using an Agilent 7700x Inductively Coupled Plasma Mass Spectrometer (ICP-MS) with collision cell. The concentrations of elements in the samples were measured within the range 0.001-25,000 mg/kg.
Veterinary Medicines
Two sub-samples of each sample were extracted using either 1% oxalic acid or 1% acetic acid in acetonitrile. After homogenising, sodium sulphate was added and the sample shaken and centrifuged at 4000-4500 rpm for 10 mins. The supernatant was then applied to dispersive C18 and / or NH2 solid phase extraction material. After further shaking and subsequent centrifugation, an aliquot of the supernatant was analysed by reverse phase ultra high performance liquid chromatography (UHPLC) coupled to tandem mass spectrometry (MS/MS) (Agilent 6490 TQ).
Pesticides
Samples were hydrated with HPLC grade water and extracted in acetonitrile, in the presence of sodium citrate, sodium chloride and magnesium sulphate. The extract was then divided with one portion mixed with dispersive SPE material (PSA and C18) before a solvent swap (into ethyl acetate) and subsequent analysis by gas chromatography with mass spectrometric detection (GC-MS, Agilent 5973 Inert MSD). The second portion was directly analysed using liquid chromatography with mass spectrometric detection (UPLC-MS/MS) in selected reaction monitoring mode (Agilent 6490 TQ). The presence of residues was confirmed using the same technique in multiple reaction monitoring mode. The concentration of pesticide residues was measured with limits of detection of 10, 20 or 50 µg/kg.
Mycotoxins
The sample was weighed into a plastic centrifuge tube. The extraction solvent used was a mixture of acetonitrile:water:acetic acid (79 : 20 : 1). Tubes were vortex mixed, then extracted for 2 hours on an orbital shaker. After extraction, tubes were centrifuged at 4000 rpm for 20 minutes at 4°C. An aliquot of the supernatant was transferred to a glass vial and diluted with a mixture of acetonitrile:water:acetic acid (20 : 79 : 1). The vials were stored overnight in a fridge at 4-8°C. Sample extracts were filtered by syringe filter (0.22 µm, nylon) and collected in glass autosampler vials for analysis.
UPLC-MS/MS analysis was carried out using a Waters UPLC system with a XEVO TQ-S mass spectrometer. Two analytical runs, one using neutral mobile phase conditions and one using acidic conditions, were required to ensure optimum chromatographic performance and ionisation of analytes. The method can detect several groups of mycotoxins including aflatoxins, fumonisins, trichothecenes, Alternaria toxins, ergot alkaloids, zearalenone and derivatives, enniatins, as well as many other Fusarium and Penicillium mycotoxins. In addition, masked forms of some mycotoxins i.e. metabolites of the parent mycotoxin, can also be detected.
PAHs
An aliquot of each homogenised sample was fortified with appropriate ¹³C internal standards and subjected to saponification followed by liquid-liquid extraction. Desired analytes were extracted from the matrix using a DMF/cyclohexane partition followed by elution through a silica gel column. Analysis was by GC-MS using a Thermo Trace Ultra GC/ISQ with a Select PAH 30m column with a 0.25 mm diameter and a 0.15 μm column film.
Nitrates and nitrites
Aliquots of each sample were extracted using water and clarified using acetonitrile. Sample extracts were chromatographed using an AS11-HC column (Thermo Scientific) with a guard column. The mobile phase was a sodium hydroxide solution with a flow rate of 1 ml/min at ambient temperature. The injection volume was 100 ul and detection was by UV. Aliquots of a reference material, with an assigned value for nitrate only, were analysed with the samples.
PFAS
Aliquots of the test samples were spiked with isotope standards (internal standards) and extracted using basic methanol. The resulting solvent extracts were solvent exchanged into water and passed through WAX SPE columns. WAX columns allow for the retention of both short and longer chain PFAS analytes due to the ionic exchange and reverse phase properties. Non-specific interferences were retained on the column whilst PFAS analytes were eluted using ammonia in methanol. Samples were concentrated and reconstituted in methanol, analysed by HPLC-MS/MS, and quantified against calibration standards of known concentrations of the PFAS (0-5 ug/kg).
Toxin screen
Each sample was extracted in triplicate with 1% acetic acid in acetonitrile by homogenisation. Anhydrous sodium sulphate was added and the mixture shaken. After centrifugation (4500 rpm, 5 min, 5oC), the supernatant was poured into a tube containing Bondesil C18 sorbent (500 mg), shaken and centrifuged (4500 rpm, 5 min, 5°C). Two aliquots were evaporated to dryness under a stream of nitrogen at 40 – 45°C. Aliquot 1 was resuspended in 1:1 methanol:0.2% aqueous acetic acid by dissolving the residue in 400 µL methanol and 100 µL internal standard mix (1 µg/mL) in methanol, vortex-mixing and adding 500 µL 0.2% aqueous acetic acid and mixing. Aliquot 2 was resuspended in 0.2% aqueous acetic acid (900 µL) and 100 µL internal standard mix (1 µg/mL) in methanol. Aliquot 1 was centrifuged (2000 rpm, 5min, 5oC) and then passed through a PTFE filter (0.2 µm). Aliquot 2 was centrifuged (14000 rpm, 2min, 5°C). Single (internal standard added) and double (100 µL methanol added) blanks were prepared in the same manner.
Portions of the extracts were analysed using an Agilent 1200 series liquid chromatograph coupled with an Agilent 6320 TOF. Chromatographic separation was achieved on a Zorbax SB-Aq (50 x 2.1mm, 1.8 µm) held at 60°C. The mobile phases comprised of water containing 0.2% acetic acid and methanol containing 0.2% acetic acid (B). The gradient started at 2% B and changed to 98% B at 13 minutes before returning to 2% B at 19 minutes. The flow rate was 0.6 mL/min and the injection volume was 2 µL. The MS was operated in positive and negative ESI with monitoring from 100 – 1600 m/z.
Sample replicate raw data files were profiled against procedural blanks using a combination of MassHunter Qualitative software (Agilent) and Mass Profiler Professional (MPP, Agilent) to produce a list of ion features present in the samples but not in the controls. This feature list was mined by comparison to an in-house database of 1250 compounds consisting of biological toxins (e.g. fyco-, myco-, phyto-). All toxins in the database are given in Appendix B for information.
Results were filtered using the following criteria:
-
Not present in the procedural blanks.
-
Present in all three sample replicates.
-
Response in MPP software > 50,000. (Represents a summation of all software-assigned ions).
-
Mass accuracy of match >-5 and <5 ppm.
-
Adduct ion match. Compounds were rejected where the spectral data indicated that the identified adduct ion was incorrect.
-
Spectral data. Compounds were rejected if the presence of atoms other than those in the compound formula are indicated (i.e. halogen), the ion charge is incorrect, or the ion corresponds to an isotopic ion of a lower mass ion.
The finalised ion list was then compared to the MetLin database to assess the likelihood of a potential compound being due to a naturally occurring metabolite in the sample.
Data was filtered on the following criteria:
- Compound rejected if there were more than 10 MetLin database matches.
Data was also reviewed against the likelihood of both the ion formed and the retention time and also against any other available information (such as for example geographical distribution of the organism producing the toxin). Finally, any compound identification with a formula match score <80% was removed.
Microbiological analysis
The microbiological analysis included organisms that are regulated for in animal feed containing animal products i.e. Salmonella spp. and Enterobacteriacae and organisms that are indicative of hygiene methods or requested by the FSA.
The microbiological content of the sample was determined following methods outlined in the following ISO standard methods:
-
ISO 4833 (2013), “Enumeration of Micro-Organisms - Colony Count Technique At 30°C (Pour Plate)”
-
ISO 21528, part 2 (2004), “Enumeration of Enterobacteriaceae”
-
ISO 4832 (2006), “Enumeration of Coliforms - Colony Count Technique”
-
ISO 6579, part 1 (2017), “Horizontal method for the detection, enumeration and serotyping of Salmonella spp.”
-
ISO 10272 part 2 (2017) and amendment 1 (2023), “Microbiology of the food chain – horizontal method for the detection and enumeration of Campylobacter” for poultry manure only
-
ISO 6888 part 1 (2021), “Microbiology of the food chain – horizontal method for the enumeration of coagulase-positive Staphylococci (Staphylococcus aureus and other species)” for catering kitchen waste and supermarket surplus only.
-
ISO 16649 part 2 (2001), “Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli”
-
Enumeration of ESBL E. coli – In-house method based on ISO 16649 part 2 (2001) using ESBL selective agar plates
-
Enumeration of Enterococci – In-house method using Slanetz & Bartley agar and the spread plate technique. Plates incubated at 37°C for 48 h. (ISO method currently under development). For frass only.
Briefly, for the colony count technique and enumeration assays the sample (10 g) was added to diluent (90 ml) and homogenised in a stomacher-style blender for 2 mins. Further dilutions (1:10) were prepared and vortexed to mix. Each dilution was used for duplicate agar plates. Agar plates were incubated as required for the method and colony numbers were counted on completion of the incubation period.
For the detection of Salmonella spp. sample (25 g) was added to pre-enrichment broth (BPW; 225 ml) and homogenised for 2 minutes in a stomacher-style blender. The sample was incubated as stated in the Standard method. Aliquots from the incubated test portion were subcultured into selective enrichment broth (RVS and MKTTn) and incubated. The selective enrichment broths were streaked onto selective agar (XLD and BGA) and incubated. When incubation was complete, the plates were examined for the presence of colonies which display a typical morphology for Salmonella spp.
Duplicate or single plates were undertaken on samples (replicates A, B1 and B2) the average log10 concentration for a replicate was estimated as the average of each log10 plate observation, then the average log10 for the sample type and source was estimated as:
Average for type and source =
An indication of within replicate variation was given by and of between replicate variation by
Results that were reported as <10 cfu/g were treated as being at the limit of detection. Results reported as being above a value were treated as being at that value.
Non-targeted virus screen
Samples were frozen at -70oC and stored prior to extraction. Each sample (2.5 g) was extracted using the CTAB based method described in Adams et al. (2009).The extracted RNA was further purified using the RNAeasy kit (Qiagen, UK) with optional on column DNAse treatment following the manufacturer’s instructions. Illumina compatible indexed DNA sequencing libraries were constructed using the truSeq Stranded RNA library kit (Illumina, UK) including the ribosome depletion steps. The libraries were indexed using the compatible IDT unique dual indexes (Illumina UK). Further details of the library preparation can be found in the instructions for the kits provided by Illumina. Equimolar quantities of the libraries were checked on a Tapestation (Agilent, UK) and pooled. The pooled library was sequenced on a 500 cycle SP NovaSeq flowcell at Newcastle University. Data was provided as demultiplexed fastq files. The sequenced data was processed using the Angua pipeline as described in Fowkes et al. (2021).
Assessment of the extent to which the results are representative of the substrate, larvae and frass
Analytical results were evaluated to provide a measure of how representative the samples for each contaminant were for each rearing substrate and to indicate the likely reason for any lack of representativeness (e.g. too few sampling times; too few primary samples; lack of homogeneity in bulk material) and how this might affect the study outcomes.
Results
The following sections highlight analytes found in larvae or, in the case of metals, those for which there are regulatory limits for presence in animal feed. The full results for each group of chemicals are provided in Appendices B-I.
Note all results, with the exception of those for microbiology, are presented on a dry weight basis.
Metals
In the UK and the EU there are regulatory limits for arsenic, cadmium, mercury and lead in animal feed (Directive 2002/32/EC) (Table 2). In the EU, there are also regulatory limits for the presence of chromium (VI), cadmium, mercury and lead in organic fertilisers (EU 2019/1009) (Table 2).
Low levels of arsenic were found in larvae reared on the baseline diets and on all four of the tested rearing substrates, but all larvae samples were below the regulatory limit (Figure 8). There was no evidence of bioaccumulation in the larvae (Appendix 2)
Cadmium was present in larvae reared on the baseline samples and on the four currently non-permitted rearing substrates tested (Figure 8). Levels were below the regulatory limits for feed materials of animal origin (2 mg/kg). The highest level was found in larvae reared on poultry manure (0.610 mg/kg). There was evidence of bioaccumulation in larvae reared on all rearing substrates (Appendix B). Bioaccumulation of cadmium by BSF larvae is known to occur when using both spiked and naturally occurring levels in rearing substrates (Addeo et al., 2024; Charlton et al., 2015; Gao et al., 2017; Purschke et al., 2017).
Low levels of lead were found in larvae reared on the baseline diets and on all four of the tested rearing substrates (Figure 8), but all larvae samples were below the regulatory limit for feed materials of animal origin. There was a low level of bioaccumulation in the larvae reared on the core diet +20%, catering surplus, manufacturing surplus, poultry manure and supermarket surplus (Appendix B). Some studies have also shown bioaccumulation of lead by BSF larvae (Addeo et al., 2024), whilst other studies have reported no bioaccumulation (Proc et al., 2020).
Mercury was not detected in any of the samples tested.
In addition to the heavy metals that have regulatory limits for their presence in feed and food, the levels of other metals are also of importance, both as micronutrients for the larvae and also for their presence in animal feed for farmed animals and pets. There was evidence for bioaccumulation of some other metals e.g. calcium. phosphorus, selenium (Appendix B). It may be necessary to assess the levels of some of these other essential metals to ensure that correct nutritional profiles are provided and that any nutritional limits are not exceeded. For example, calcium and phosphorus are essential elements for nutrition and are required in, for example, poultry feed for bone and muscle growth and eggshell formation together with other functions. However, too much calcium can reduce growth rates in broiler and layer pullets, whilst an oversupply of phosphorus can have an environmental impact when manure is applied to soils.
Regulation (EU) 2019/1009 specifies the following criteria for solid organic fertilisers:
Contaminants in an organic fertiliser must not exceed the following limit values:
-
cadmium (Cd): 1.5 mg/kg dry matter,
-
hexavalent chromium (Cr VI): 2 mg/kg dry matter,
-
mercury (Hg): 1 mg/kg dry matter,
-
nickel (Ni): 50 mg/kg dry matter,
-
lead (Pb): 120 mg/kg dry matter, and
-
inorganic arsenic (As): 40 mg/kg dry matter.
The copper (Cu) content in an organic fertiliser must not exceed 300 mg/kg dry matter and the zinc (Zn) content in an organic fertiliser must not exceed 800 mg/kg dry matter.
These levels were not exceeded in any of the frass samples tested (Appendix B).
Veterinary Medicines
The veterinary medicines method screens for 115 compounds. Traces of eight compounds were detected in at least one extract from one sample type (Figure 9). Full results are shown in Appendix C.
Nicarbazin is a coccidiostat and was detected in the poultry manure rearing substrate, the larvae that were produced and the resulting frass (Figure 9). Levels in the larvae were much lower than those found in the rearing substrate or the frass (means 5775, 363.8 and 2725 µg/kg respectively). Low levels of nicarazin were also found in the catering waste (mean of 12.25 µg/kg) and the frass produced from the catering waste (mean of 36.25 µg/kg), but was not detected in the larvae reared on the catering waste.
The regulatory limit for the presence of nicarbazin in feed materials relative to a feed with a moisture content of 12% is 1.25 mg/kg. Therefore, although the level of nicarbazin in the larvae reared on the poultry manure would not exceed the regulatory limit for a feed material, the level present in the poultry manure rearing substrate does exceed the regulatory limit if larvae are classed as a farmed animal and this substrate were to be provided as feed.
Flubendazole is an anthelmintic used as a wormer in poultry. It was not detected in the rearing substrates or the frass but was detected in larvae reared on the manufacturing surplus (mean 20.5 µg/kg) (Figure 9). It is possible that as the larvae would have greater homogeneity than either the substrate or the frass, this increased the likelihood of detecting this compound. Alternatively, the larvae may have bioaccumulated the compound from the substrate to a level where it could be detected. Further research would be needed to determine whether bioaccumulation of this compound does occur. There is no regulatory limit for this compound in feed ingredients.
Lasalocid is an antibiotic and coccidiostat used as a feed additive. Lasalocid was detected in larvae reared on manufacturing surplus (mean 86.9 µg/kg) (Figure 9). The regulatory limit for lasalocid in feed materials is 1.25 mg/kg and therefore the regulatory limit was not exceeded in these samples.
Narasin and salinomycin were detected at very low levels in larvae reared on poultry manure (means of 20.25 µg/kg and 14.75 µg/kg respectively). These levels were much lower than those detected in the poultry manure rearing substrate (means of 3562 µg/kg and 3875 µg/kg for narasin and salinomycin respectively) or in the frass (means of 385 µg/kg and 195 µg/kg respectively) (Figure 9). Both compounds have a regulatory limit of 0.7 mg/kg in feed materials. The level of these compounds in the larvae would not exceed the regulatory limit for a feed material, however, the level present in the rearing substrate does exceed the regulatory limit if larvae are classed as a farmed animal and this substrate were to be provided as feed.
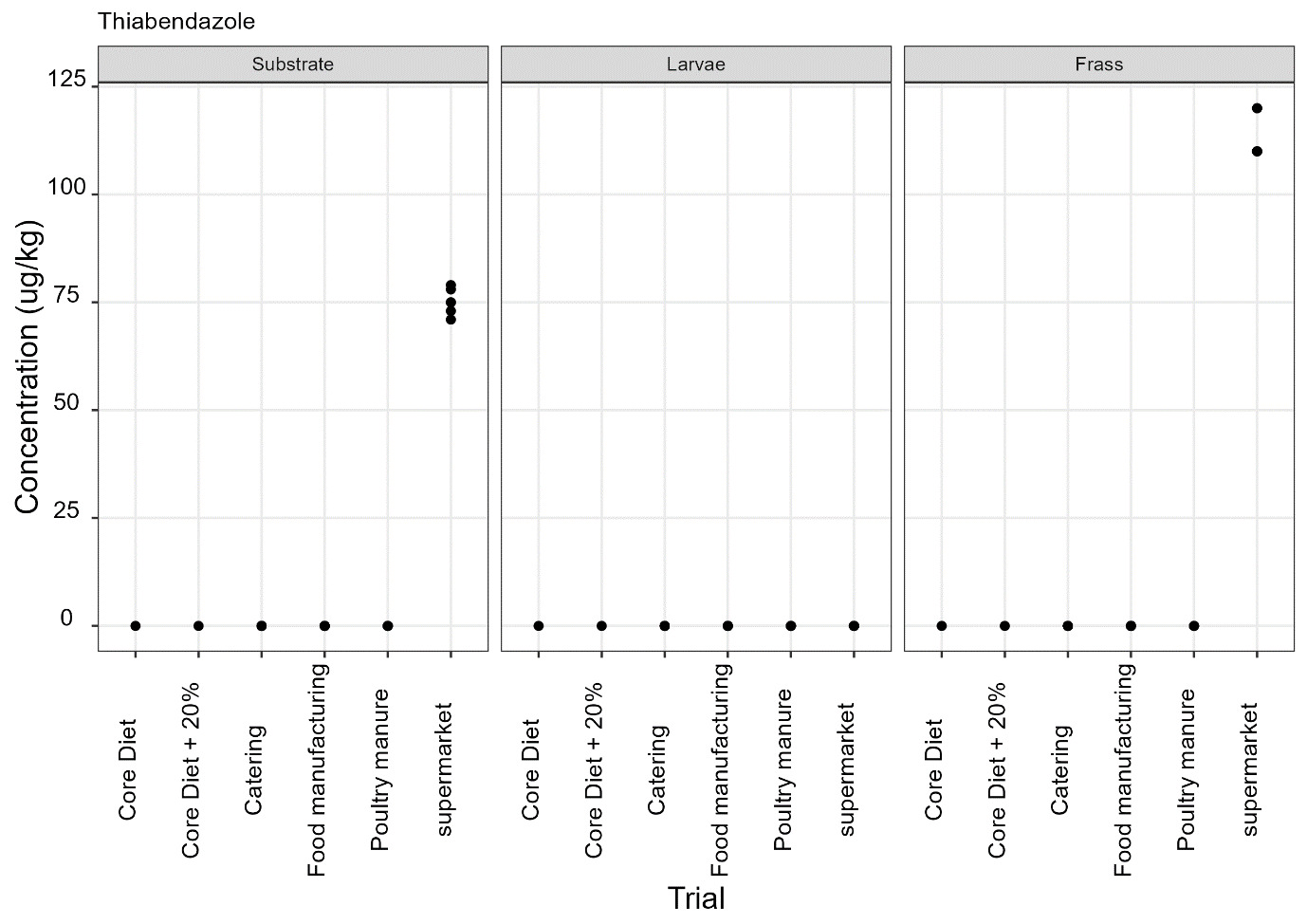
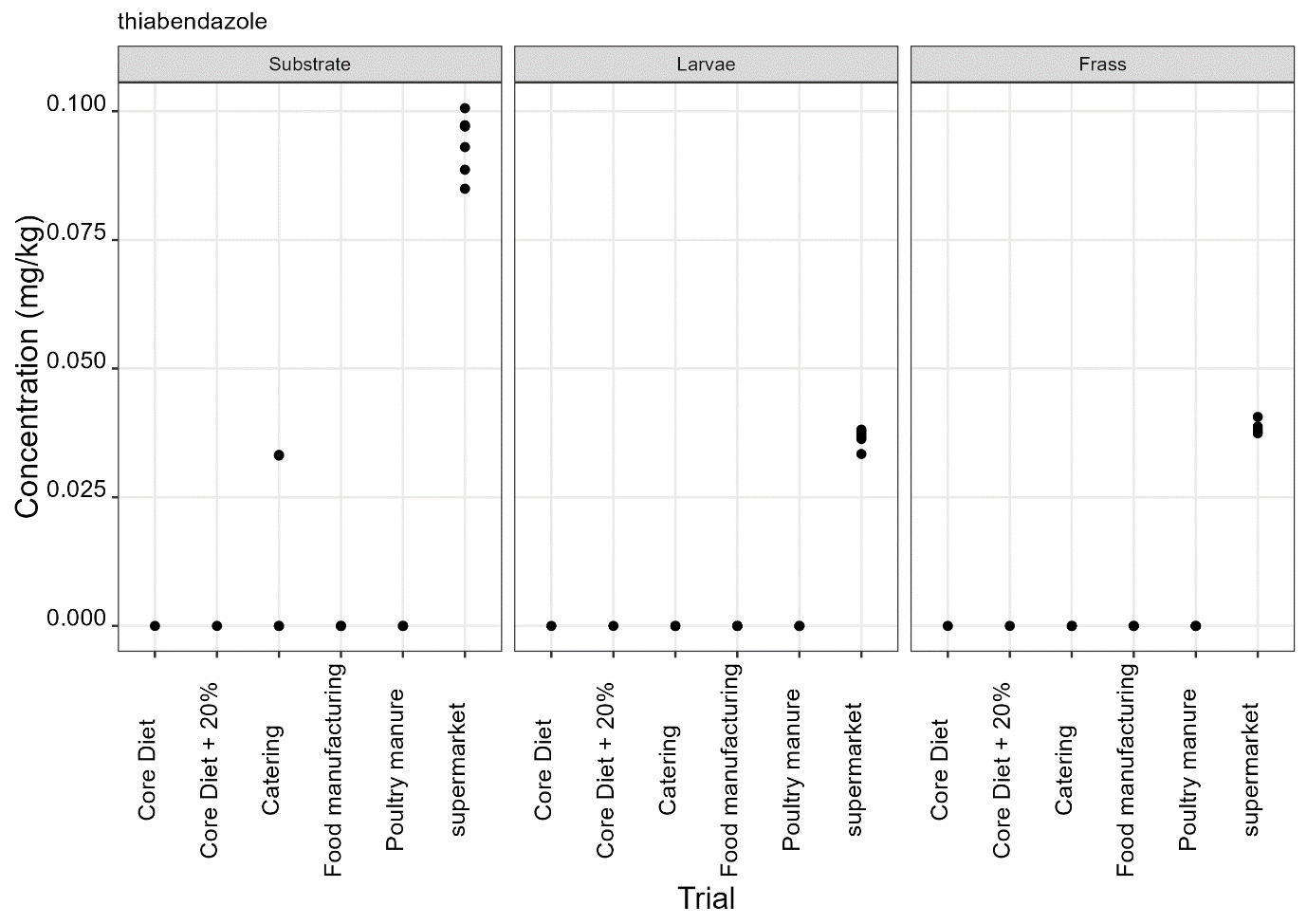
Thiabendazole, triclabendazole sulphoxide and 5-hydroxythiabendazole are anthelmintics used to treat parasitic worms. Thiabendazole and triclabendazole sulphoxide were detected in the supermarket surplus rearing substrate (means of 74.4 µg/kg and 20.1 µg/kg respectively) and frass (means of 112.5 µg/kg and 12.38 µg/kg respectively) but were not detected in the larvae reared on supermarket surplus (Figure 9). 5-hydroxythiabendazole was detected in the frass from supermarket surplus (mean of 70.9 µg/kg) but was not detected in the rearing substrate (Figure 9). It is considered that the frass may be more homogenous than the rearing substrate due to the activity of the larvae and this may increase the likelihood of detection of this compound at low levels. There are no regulatory limits for these compounds in animal feed.
Pesticides
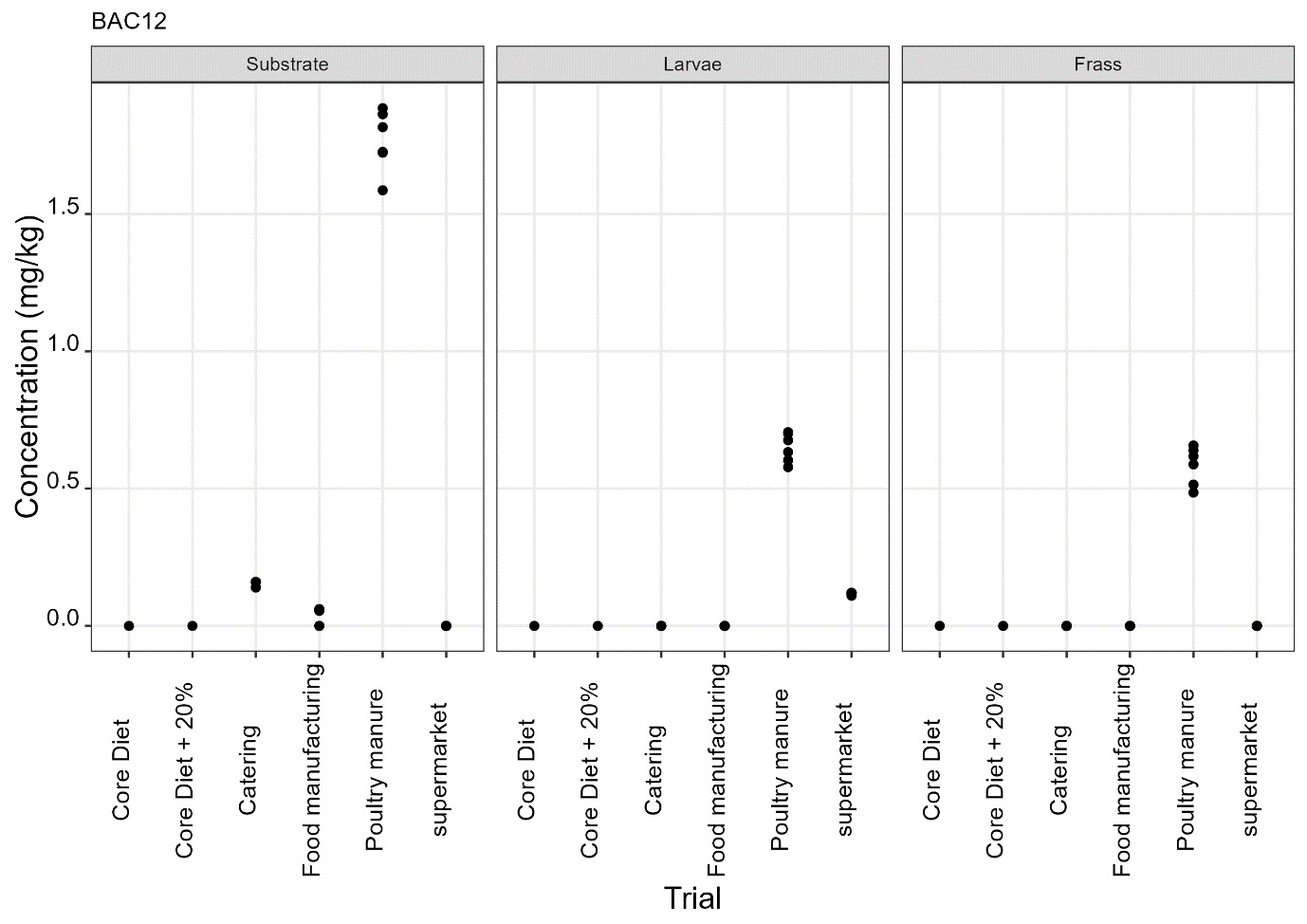
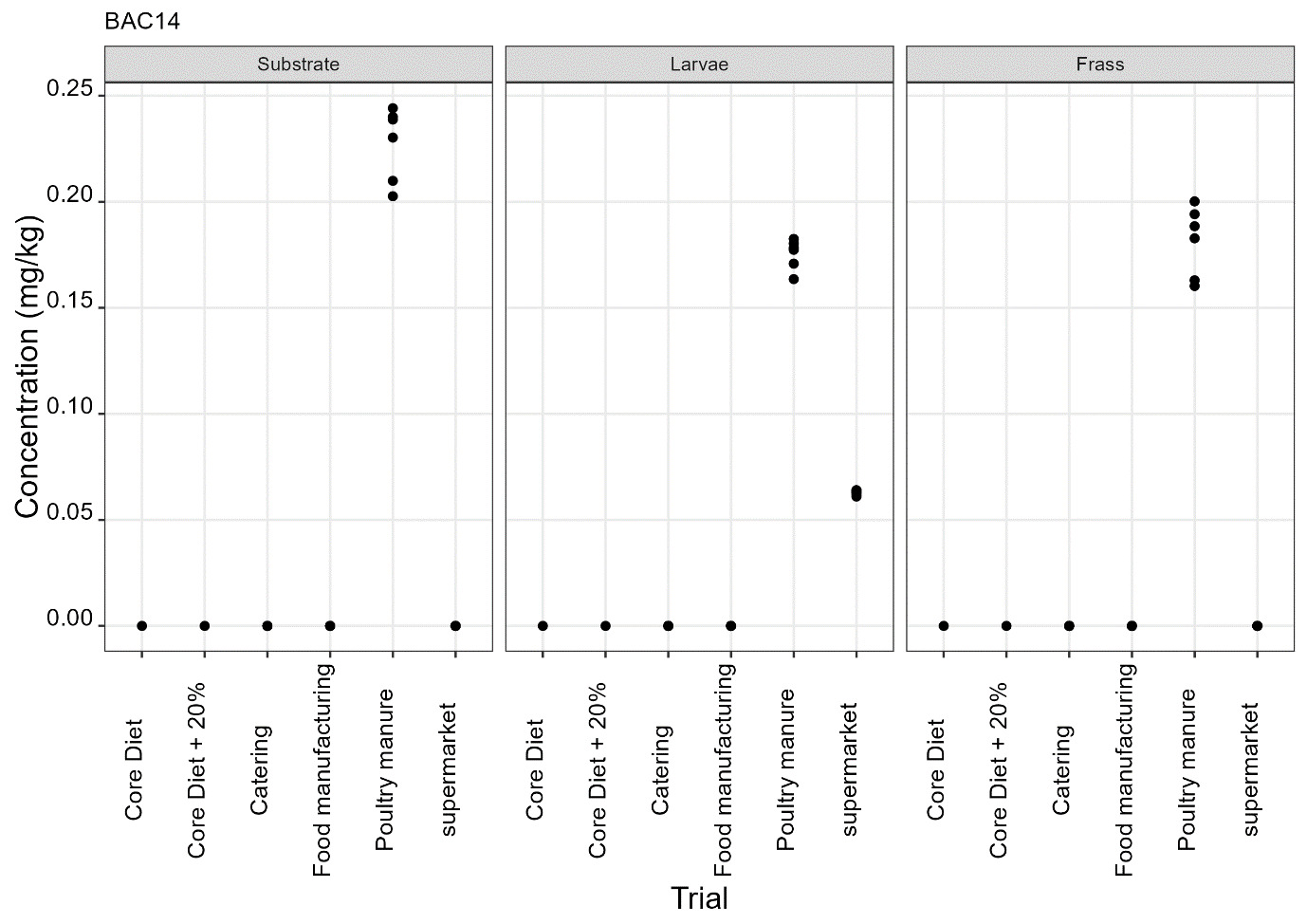
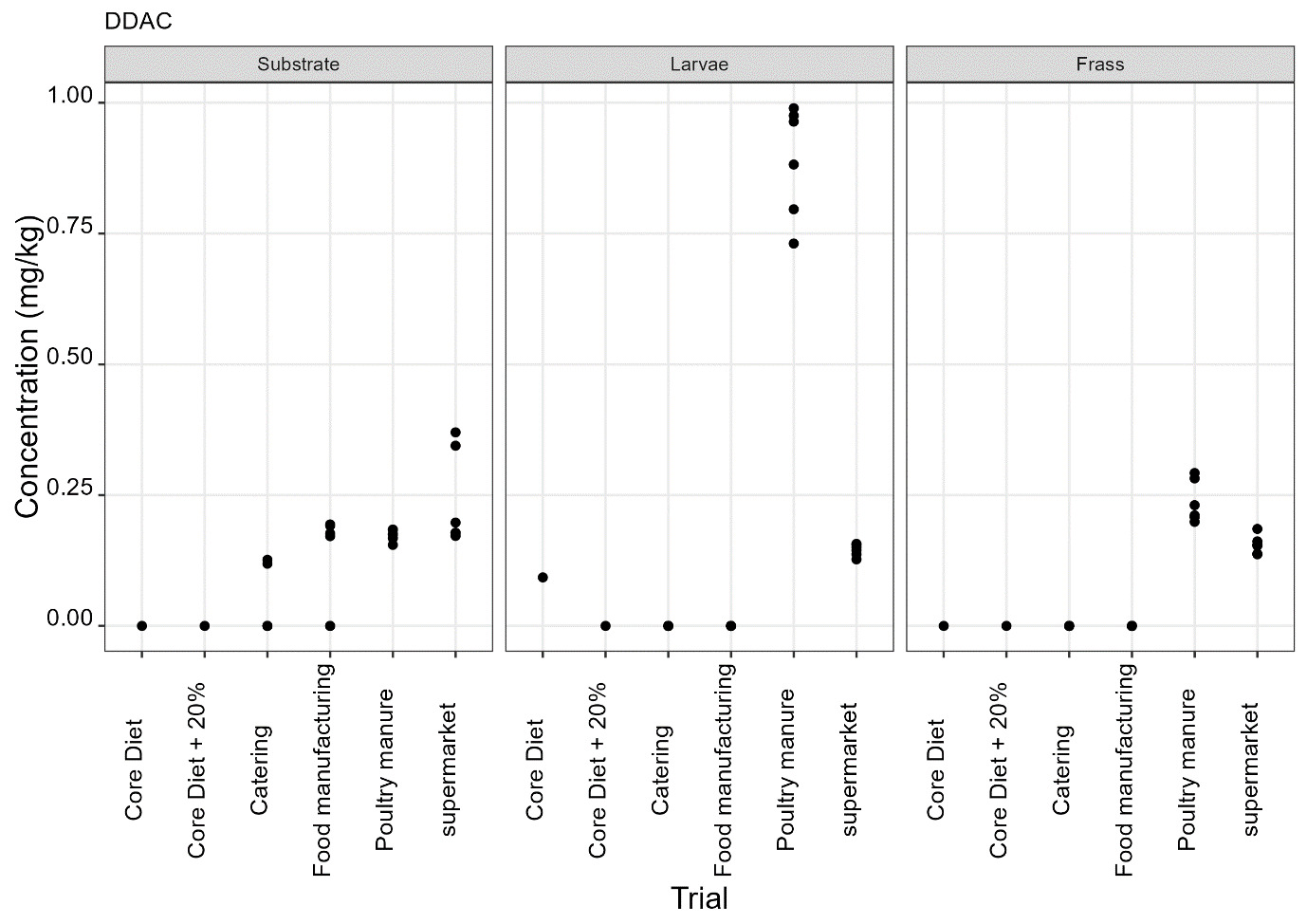
The pesticides analysis screens for 427 compounds. Eleven of these compounds were detected in larvae and the majority of these were reared on either poultry manure or supermarket surplus (Figure 10). With one exception, the pesticide found in the larvae was also present in the rearing substrate. The exception was the presence of DDAC in larvae reared on core diet where DDAC was detected at 0.09 µg/kg in a single injection. Full results are provided in Appendix D.
Compounds that were detected in the larvae were:
-
BAC 12, BAC 14 and DDAC are quaternary ammonium compounds widely used as biocides for the cleaning and sanitation of surfaces in food production and for surface cleaning of milking equipment and milk storage tanks.
-
2-phenylphenol is a biocide used as a surface disinfectant.
-
Diphenylamine, fludioxonil, imazalil, and pyrimethanil are fungicides.
-
Haloxyfop and 2,4-D are herbicides.
None of these compounds have regulatory limits in animal feed ingredients.
It was noted that the levels of DDAC in larvae reared on core diet and poultry manure (means of 0.09 µg/kg and 0.9 µg/kg) were greater than in the rearing substrate (not detected and mean of 0.175 µg/kg in core diet and poultry manure respectively). However, this was not observed for larvae reared on other types of rearing substrate (catering, manufacturing and supermarket surplus).
A greater level of haloxyfop in larvae reared on poultry manure (mean of 0.03 µg/kg) compared to the poultry manure rearing substrate (mean of 0.01 µg/kg) was also noted. This may be an indication of bioaccumulation, but further research would be needed to ascertain if this is the case.
There has been less research on the uptake of pesticides by BSF larvae than for heavy metals and mycotoxins but studies have shown that the selected pesticides assessed do not bioaccumulate in BSF larvae (chlorpyrifos, chlorpyrifos-methyl, pirimiphos methyl (Purschke et al., 2017); chlorpyrifos, propoxur, cypermethrin, imidacloprid, spinosad, tebufenozide (Meijer et al., 2021)). However, in a study using almond hulls as the rearing substrate it was demonstrated that bifenthrin bioaccumulated in BSF larvae, whilst there was no bioaccumulation of other pyrethroids present in the almond hulls (Li & Bischel, 2022).
There were an additional nine compounds that were detected in one or more of the rearing substrates that were not detected in the larvae and two compounds that were found only in the frass of one of the rearing substrates (Appendix D).
Maximum levels for pesticides are specified in the EU Directive on Undesirable Substances and Products (Directive 2002/32/EC) and in the EU Regulation on Pesticide Maximum Residue Limits (MRLs) (Regulation (EC) No 396/2005).
The levels of the pesticides found in larvae did not exceed the MRLs for these compounds for products exclusively used for animal feed production, but some did exceed the MRLs for terrestrial invertebrate animals (Table 3). This may have implications if the larvae were to be used as food.
Mycotoxins
The mycotoxin analysis screens for 72 compounds. Results for all compounds are provided in Appendix E. Two compounds were detected in larvae: roquefortine C in larvae reared on the core diet (mean of 5.2 µg/kg) and wortmannin in larvae reared on manufacturing surplus (mean of 86.6 µg/kg) (Figure 11). There are no regulatory limits for these compounds in animal feed.
Roquefortine C is a relatively common mycotoxin produced by a number of Penicillium species. It has neurotoxic properties at high concentrations and has been found in levels between 0.05 and 1.47 mg/kg in blue cheeses and at these levels the authors considered that this was not hazardous for consumers (Finoli et al., 2001).
Wortmannin is a metabolite produced by Fusarium oxysporum, Penicillium funiculosum and P. wortmannii. It is an inhibitor of phosphoinositide 3-kinase and can disrupt signalling pathways and has high mammalian cytotoxicity.
Both roquefortine C and wortmannin were detected in the larvae but not in the rearing substrate. Although this could be an indication of bioaccumulation, the variation between samples (see later section) could also be a factor in this result. Further studies would be needed to ascertain whether there is any bioaccumulation of these compounds. Generally, it is considered that for the mycotoxins that have been investigated there is no bioaccumulation, but there are a relatively low number of mycotoxins that have been studied (Lievens et al., 2021).
A further seven mycotoxins were detected in either the rearing substrate or the frass, but were not detected in the larvae. These were:
-
Beauvericin in poultry manure substrate and frass
-
Cyclopiazonic acid in catering surplus substrate
-
Enniatin A1 in poultry manure substrate
-
Enniatin B in core diet + 20%, poultry manure and supermarket surplus substrates and supermarket surplus frass
-
Enniatin B1 in core diet + 20%, poultry manure and supermarket surplus substrates and poultry manure and supermarket surplus frass
-
Moniliformin in manufacturing and supermarket surplus rearing substrates
-
Penicillic acid in supermarket surplus rearing substrate and frass
PAHs
PAHs can be found in food that has been smoked or cooked using grilling, frying or charbroiling. On the basis of likely introduction of PAHs to the rearing substrates used, the presence of PAHs was therefore only assessed for the catering waste. Of the 30 compounds included in the analysis eight PAH compounds were found in larvae in at least one sample (Figure 12). These compounds were also detected in the rearing substrate and the frass. Full results are provided in Appendix F.
The most important PAHs are considered to be Benzo(a)pyrene, benzo(a)anthracene, benzo(b)fluoranthene and chrysene and collectively their sum is known as PAH4. The PAH4 sum lower and upper for the rearing substrate, larvae and frass are shown in Tables 4 - 6.
There are no maximum limits specified for PAHs in animal feed. There are limits for the PAH4 sum concentration in some foods, for example, baby food has a limit of < 1 µg/kg (Retained EU regulation No. 835/2011).
There was high variation between samples for some compounds and therefore the likelihood of bioaccumulation for some of these compounds was difficult to assess.
Nitrates and nitrites
The levels of nitrates and nitrites found in the rearing substrates, larvae and frass are shown in Figure 13 and the full results are provided in Appendix G. There is a regulatory limit of 15 mg/kg for nitrite in feed materials (EU Directive 2002/32/EC). This level was not exceeded in the larvae from any of the rearing substrates and there was no evidence of bioaccumulation in the larvae (Appendix G).
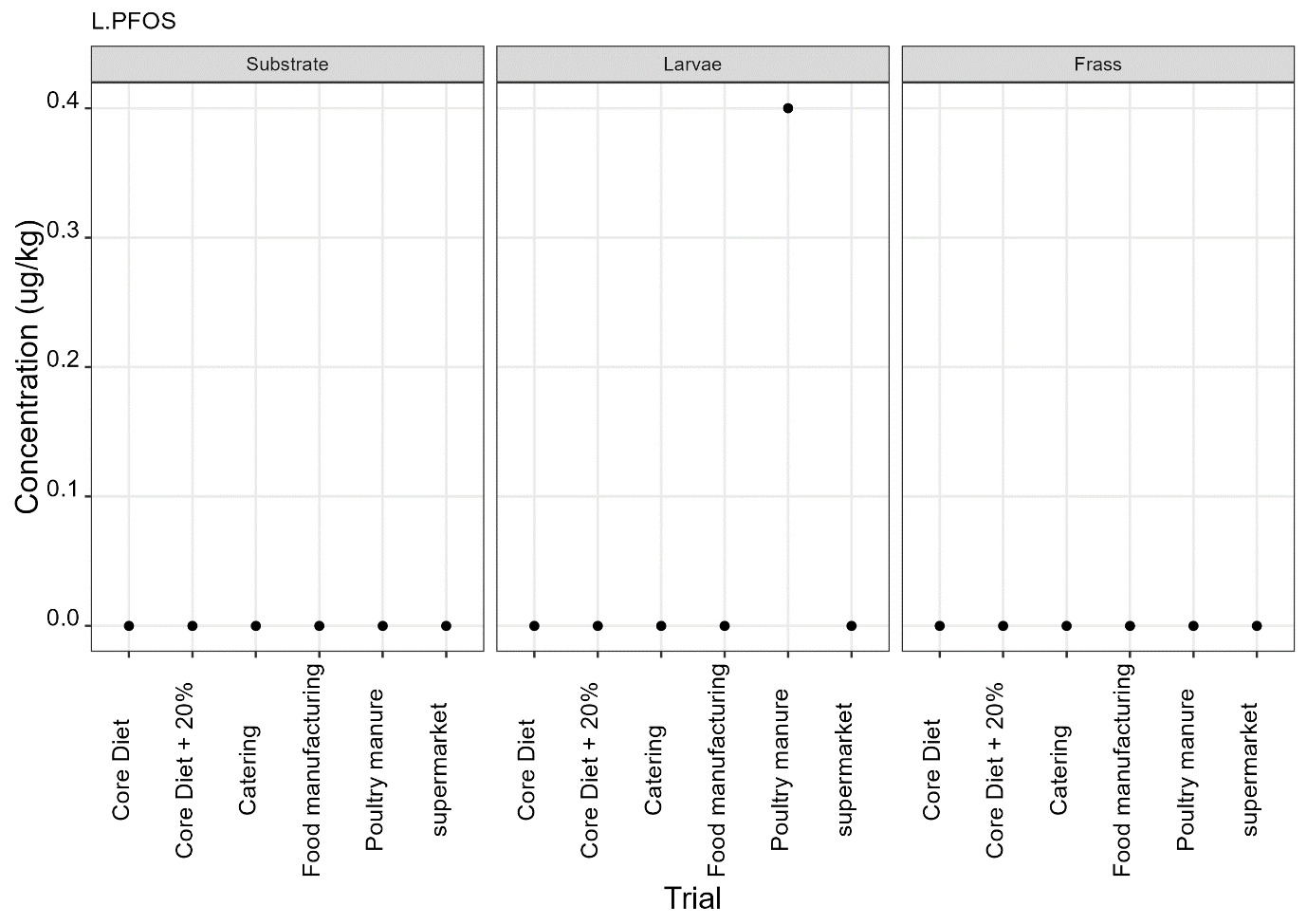
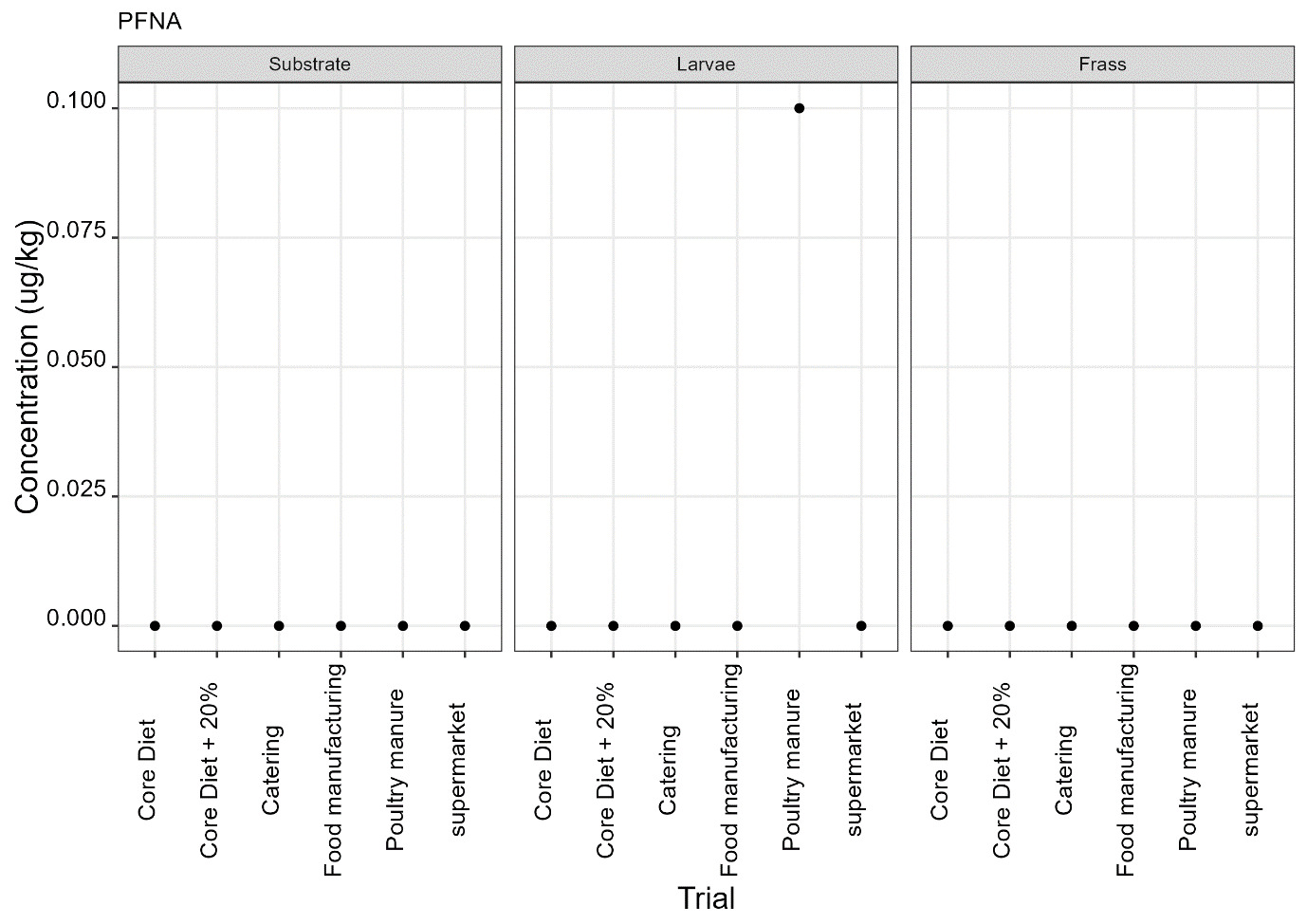
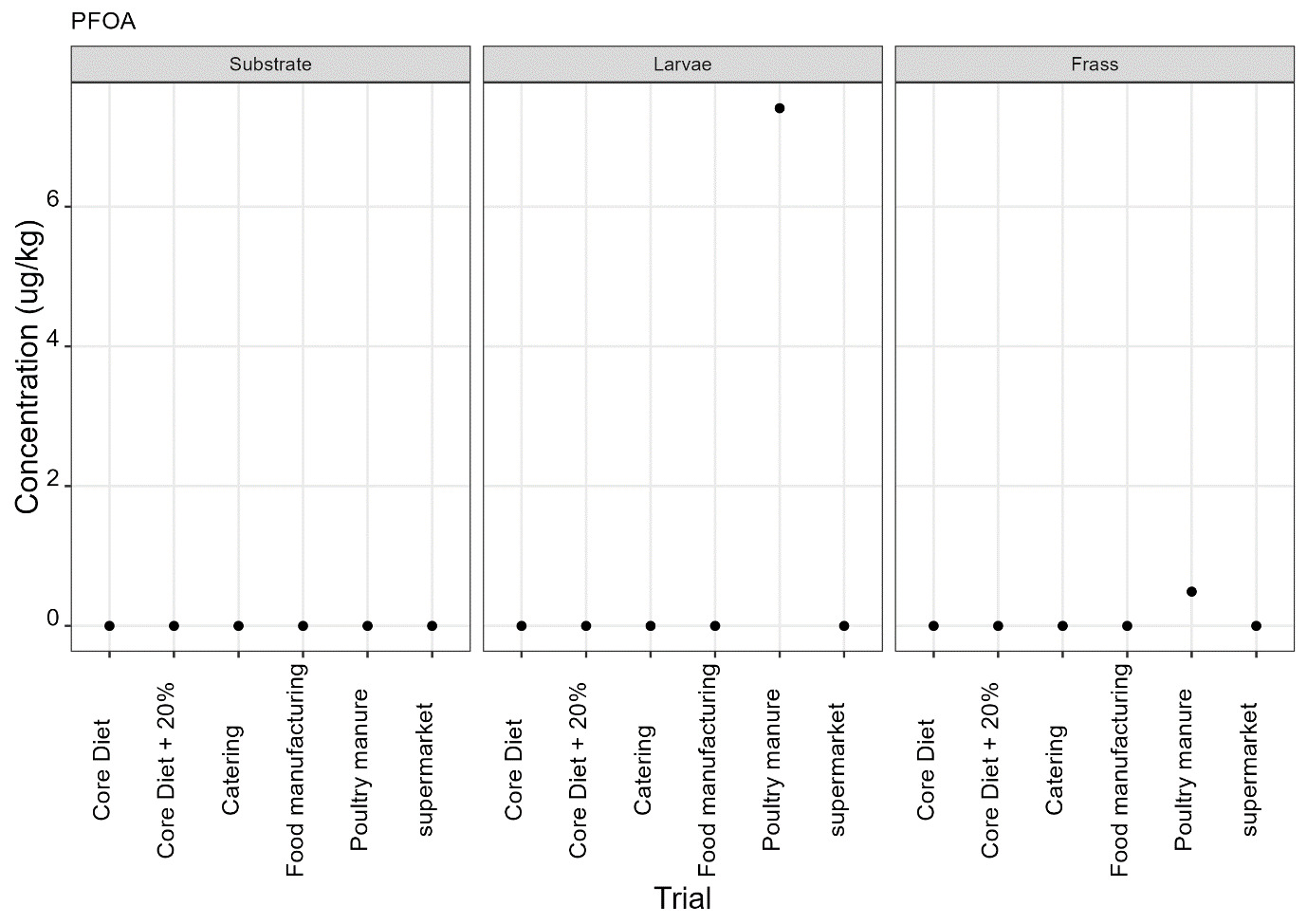
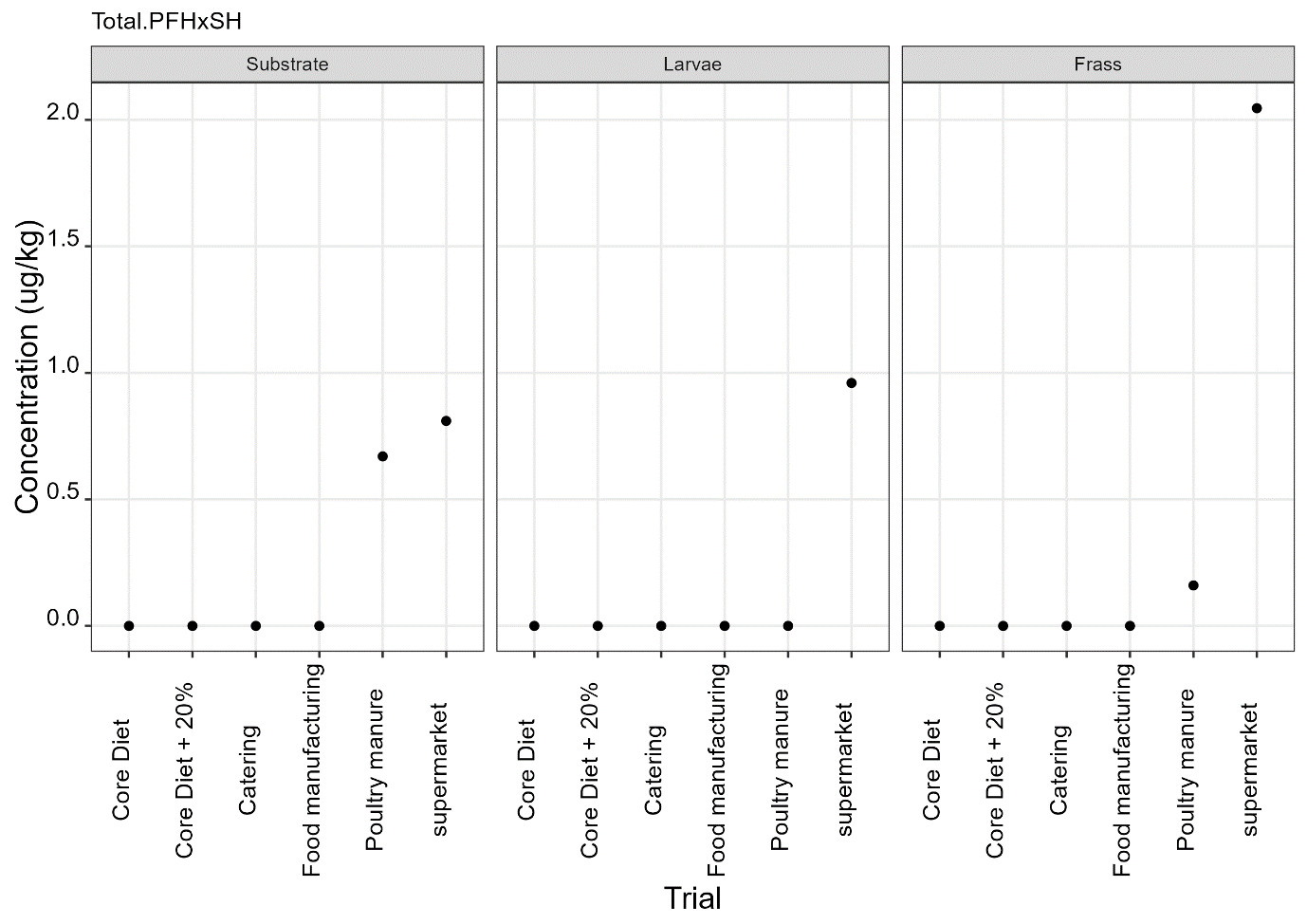
PFAS
PFAS compounds were detected in the rearing substrates, larvae and frass. It was found that some extracts were very complex resulting in low and inconsistent recoveries for some of the analytes. Chromatographic retention time shift was seen for rearing substrate and sometimes the larvae samples. Three of the analytes (PFDoA, PFTrDA and PFTeDA were not possible to integrate due to retention time shift. Some compounds were found in the frass but not in the rearing substrate or the larvae. This may be due to the complexity of the samples or due to greater homogeneity of the frass compared with the rearing substrate due to the activity of the larvae.
Compounds found in larvae are shown below (Figure 14) and the full results are provided in Appendix H.
Currently there are no regulatory limits for PFAS in animal feed but in the EU Commission Regulation 2023/915 specifies maximum limits for PFAS in animal products. This regulation sets maximum limits for PFOS, PFOA, PFNA and PFHxS individually and for their sum in meat, fish products, crustaceans and bivalve molluscs and eggs. fishery products, meat and eggs. These levels range from 0.2 to 50 µg/kg depending on the food and the compound. In this study the highest level of any PFAS compound found in larvae was for PFOA in larvae reared on poultry manure with a mean of 7.4 µg/kg. All other compounds had levels in larvae of below 0.7 µg/kg.
In this study, only a single replicate was analysed for each substrate and source and this should be considered when looking at the data. However, for some compounds levels found in the larvae were slightly higher than in the substrate e.g. HFPO.DA in larvae from manufacturing waste (substrate 0.14 µg/kg; larvae 0.20 µg/kg) and BrPHFX in larvae from supermarket surplus (substrate 0.49; larvae 0.68) (Appendix H).
Li and Bischel (2022) reported that PFBA, PFOA, PFBS and PFOS did not bioaccumulate in BSF larvae. Their study used almond hulls spiked with PFBA, PFOA, PFBS, and L-PFOS. BSF larvae were added to the spiked feed when 5-days-old and were reared on the spiked substrate for 14 days (Li and Bischel., 2022). Further studies on a wider range of these chemicals are required to ascertain whether bioaccumulation in larvae can occur.
Toxin screen
Data Criteria
Following profiling and database matching of the samples against procedural blanks, the data was scored against the criteria summarised below (Table 7). A maximum score of 10 is possible. Normally only compounds scoring greater than or equivalent to 5 are assessed as being of potential interest or reported as tentative identities (IDs).
Core Diet - Rearing substrate sample
Two features were scored as 9 or 10 (identity confirmed versus reference material). These were identified as:
-
Solanidine. This is an alkaloid produced by plants of the family Solanaceae (including potato, tomato, egg plant). The concentration was estimated by reference to an external standard to be in the range 900 – 1620 µg/kg.
-
α-chaconine. This is a glycoside of solanidine produced by plants of the family Solanaceae (including potato, tomato, egg plant). Identification was supported by the detection of three in-source fragments also found in the reference standard. The concentration was estimated by reference to an external standard to be in the range 650 – 1160 µg/kg.
One feature presented as two peaks was scored 5 and 6 for the individual peaks. This was tentatively identified as:
- Glycyrrhetinic acid. This is the aglycone of glycyrrhizin (the chief sweet-tasting constituent of liquorice root (Glycyrrhiza glabra). Although commonly used in flavouring, excessive consumption can result in hypertension and irregular heart rhythm and in extreme cases death.
Two additional features were scored as 5. These were tentatively identified as:
-
Urushiol I. This is a catechol phytotoxin with allergenic properties, causing an allergic dermatitis reaction. It is usually present as part of a mixture of related compounds. It is found in a number of species, for example poison ivy, mango, and cashew.
-
Emodin. This is classified as both a phytotoxin and a mycotoxin. It can be isolated from, for example, rhubarb and buckthorn and also from many species of fungi. However, emodin was not detected in the mycotoxin screen for this rearing substrate. This may be due to the use of different sub-samples in the different tests, but the toxin screen score of 5 also indicates a tentative identification, which would need further investigation using a known standard.
Core Diet larvae sample
One feature was scored as 9 (identity confirmed v reference material). This was identified as solanidine. The concentration was estimated by reference to an external standard to be in the range 1404 – 2229 µg/kg.
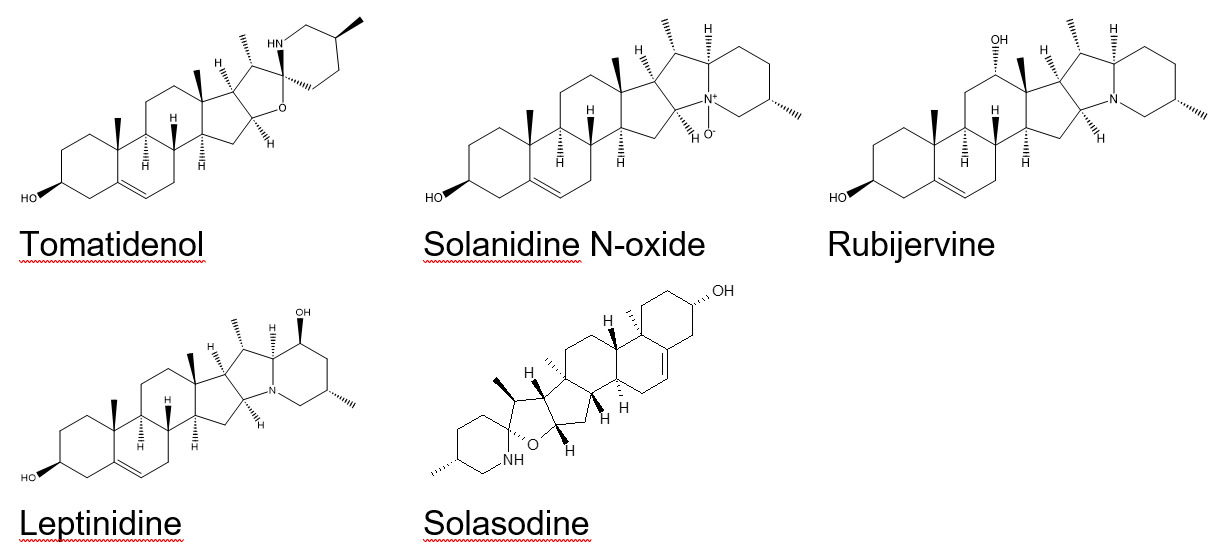
One feature presented as three peaks, each scored 7 (strong indication of identity in the absence of reference standard) respectively. Five possible related compounds were tentatively identified (see Figure 15), with no clear evidence as to which corresponds to which peak:
-
Tomatidenol
-
Solanidine N-oxide
-
Rubijervine
-
Leptinidine
-
Solasodine
These five compounds are also produced by plants of the family Solanaceae and may be reasonably expected to occur alongside solanidine.
Core diet frass sample
Two features were scored as 9 or 10 (identity confirmed v reference material). These were identified as solanidine (concentration estimated by reference to an external standard to be in the range 1404 – 1513 µg/kg) and α-chaconine (identification was supported by the detection of three in-source fragments also found in the reference standard). The concentration of α-chaconine was estimated by reference to an external standard to be in the range 632 – 681 µg/kg.
Two features were scored as 8 (strong indication of identity in the absence of reference standard). These were identified as γ-chaconine (alternative ID γ-solanine) and ß1-chaconine (alternative IDs ß1-solanine, ß2-chaconine, ß2-solanine).
One feature presented as three peaks, each were scored 6 or 7 (strong indication of identity in the absence of reference standard) respectively. There were five possible related compounds tentatively identified (tomatidenol, solanidine N-oxide, rubijervine, leptinidine and Solasodine; see Figure 15), with no clear evidence as to which corresponds to which peak. All these compounds are also produced by plants of the family Solanaceae and may be reasonably expected to occur alongside solanidine and α-chaconine.
Two features were scored as 5. These were tentatively identified as emodin and glycyrrhetinic acid.
Core diet +20% rearing substrate sample
Two features were scored as 9 or 10 (identity confirmed v reference material). These were identified as solanidine (concentration to be in the range 473 – 996 µg/kg) and α-chaconine (concentration to be in the range 227 – 478 µg/kg).
One feature was scored as 6. This was tentatively identified as cicutoxin with an alternative ID of oenanthotoxin. These two compounds are isomeric and are classified as phytotoxins. They are neurotoxins causing death by respiratory paralysis resulting from disruption of the central nervous system. They are produced by members of the family Apiaceae (includes water hemlock, water dropwort), which are found in North America and parts of Europe.
Two features were scored as 5. These were tentatively identified as urushiol I and emodin.
Core diet + 20% larvae sample
One feature was scored as 9 (identity confirmed v reference material). This was identified as solanidine. The concentration was estimated by reference to an external standard to be in the range 813 – 1774 µg/kg.
One feature presented as two peaks, each was scored 6 or 7 (strong indication of identity in the absence of reference standard) respectively. There were five possible related compounds tentatively identified (tomatidenol, solanidine N-oxide, rubijervine, leptinidine and solasodine; see Figure 15), with no clear evidence as to which corresponds to which peak.
One feature was scored as 5. This was tentatively identified as fusaric acid, a mycotoxin produced by Fusarium species.
Core diet + 20% frass sample
Two features were scored as 9 or 10 (identity confirmed v reference material). These were identified as solanidine (concentration estimated to be in the range 1492 – 2325 µg/kg) and α-chaconine (concentration estimated to be in the range 1275 - 1995 µg/kg).
One feature was scored as 8 (strong indication of identity in the absence of reference standard). This was identified as γ-chaconine (alternative ID γ-solanine).
One feature presented as two peaks; each was scored as 7 (strong indication of identity in the absence of reference standard). There were five possible related compounds tentatively identified (tomatidenol, solanidine N-oxide, rubijervine, leptinidine and solasodine; see Figure 15), with no clear evidence as to which corresponds to which peak.
One feature was scored as 6. This was tentatively identified as cicutoxin (alternative ID oenanthotoxin).
Three features were scored as 5. These were tentatively identified as fusaric acid, emodin and flavipucine, classified as a mycotoxin.
Currently permitted rearing substrates: Results of note
Two glycoalkaloids solanidine and alpha-chaconine associated with plants of family Solanaceae (potato, tomato, eggplant etc) could be identified by comparison with a reference material. Residue levels in freeze-dried samples ranged from estimated 0.2 to 2.3 mg/kg. The solanine levels increased in the larvae and frass, compared to the substrate.
Alpha-chaconine levels were lower than the solanidine level in the same sample; larvae samples did not contain any alpha-chaconine.
The related compounds tomatidenol/solanidine N-oxide, rubijervine, leptinidine, solasodine, and gamma-chaconine associated with plants of the family Solanaceae were also tentatively identified.
The other five compounds (cicutoxin, urushiol I, emodin, fusaric acid, flavipucine) tentatively identified may warrant further investigation.
Currently there are no maximum levels of glycoalkaloids specified for animal feed. A risk assessment published by EFSA concluded that a risk characterisation of potato glycoalkoloids in feed for farm and companion animals was not possible due to insufficient data on potential adverse effects in these species (EFSA, 2020). Data on the presence of these compounds in animal feed is therefore required before an assessment of the levels found in insect larvae can be evaluated.
Currently non-permitted substrates
Catering rearing substrate sample
One feature was scored as 6 (strong indication of identity in the absence of reference standard). This was tentatively identified as sparteine. This is an alkaloid phytotoxin found in Cytisus scoparius (Common broom) and also in species of lupin (Lupinus genus). It acts as a sodium channel blocker.
Three additional features were scored as 5. These were tentatively identified as:
-
1,4-ipomeadiol - found in mould-damaged sweet potatoes (Ipomoea batatas). Known to cause pulmonary toxicity in cattle and rodents.
-
1-(3’-furyl)-6 7-dihydroxy-4 8-dimethylnonan-1-one - found in mould-damaged sweet potatoes.
-
Aposcopolamine - a tropane alkaloid found in members of the Nightshade family (Solanaceae), in particular Datura ferox (Angel’s trumpets, fierce thornapple). An alternative identification for this feature was morphine (an opioid alkaloid).
Catering larvae sample
One feature was scored as 6 (strong indication of identity in the absence of reference standard). This was tentatively identified as muscimol. This is a psychoactive component of mushroom species such as fly agaric (Amanita muscaria).
Three additional features were scored as 5. These were tentatively identified as:
-
Aposcopolamine.
-
Fusaric acid - a mycotoxin produced by Fusarium species.
-
Penigequinolone A - a mycotoxin produced by Pencillium spp.
Catering frass sample
One feature was scored as 9 (identity confirmed v reference material data). This was identified as alpha-chaconine.
One feature was scored as 7 (strong indication of identity in the absence of reference standard). This was tentatively identified as:
- Muscimol
Three features were scored as 6 (strong indication of identity in the absence of reference standard). These were tentatively identified as:
-
Anagyrine - a phytotoxin produced by a number of plant species including members of the genus Lupinus (lupins) and Anagyris foetida (stinking bean trefoil). An alternative identification of verruculotoxin (a mycotoxin produced by Penicillium verrucosum) was scored as 5.
-
Aposcopolamine.
-
Flavipucine - a mycotoxin.
An additional seven features were scored as 5. These were tentatively identified as:
-
Aphidicolin - a mycotoxin.
-
Chanoclavine - an ergot alkaloid. Alternative identifications for this feature were the ergot alkaloids isochanoclavine, dihydroelymoclavine, dihydroisolysergol, dihydrolysergol and fumigaclavine B and the roquefortine alkaloid roquefortine B, all scored as 5.
-
Chlamydosporol - a mycotoxin.
-
Deoxaphomin - a mycotoxin.
-
Fusaric acid - a mycotoxin produced by Fusarium species.
-
Indole-3-acetic acid - a plant hormone. This has been listed as mutagenic and potentially carcinogenic.
-
1-ipomeanol - found in mould-damaged sweet potatoes (Ipomoea batatas).
Manufacturing rearing substrate sample
Two features were scored as 7 (strong indication of identity in the absence of reference standard). These were tentatively identified as:
-
1,4-ipomeadiol - Found in mould-damaged sweet potatoes (Ipomoea batatas). Known to cause pulmonary toxicity in cattle and rodents.
-
Galantamine - a phytotoxin found in Narcissus spp (e.g. daffodil). An alternative identification, also scored a 7, was pluviine, an isomeric phytotoxin also found in Narcissus spp.
One feature was scored as 6 (strong indication of identity in the absence of reference standard). This was tentatively identified as:
- Seneciphylline - a pyrrolizidine alkaloid found in e.g. Jacobaea vulgaris (ragwort). An alternative identification also scored as 6 was spartioidine, also a pyrrolizidine alkaloid found in e.g. Jacobaea vulgaris (ragwort).
An additional five features were scored as 5. These were tentatively identified as:
-
Aposcopolamine.
-
Agistatin A - a mycotoxin.
-
Juglone - a phytotoxin produced by members of the Juglandaceae family (walnuts). Toxic to other plant species.
-
Muscimol.
-
Tryprostatin A - a mycotoxin.
Manufacturing larvae sample
One feature was scored as 7 (strong indication of identity in the absence of reference standard). This was tentatively identified as:
- Heliotrine - a pyrrolizidine alkaloid phytotoxin produced by plants of the genus Heliotropium.
One feature was scored as 6 (strong indication of identity in the absence of reference standard). This was tentatively identified as:
- Fusaric acid
An additional three features were scored as 5. These were tentatively identified as:
-
1,4-ipomeadiol.
-
Aposcopolamine.
-
Prop-2-ene carboxylic acid. This compound is a mycotoxin found in some mushrooms such as Russula subnigricans.
Manufacturing frass sample
One feature was scored as 6 (strong indication of identity in the absence of reference standard). This was tentatively identified as:
- Fusaric acid.
An additional five features were scored as 5. These were tentatively identified as:
-
1-myoporol - found in mould-damaged sweet potatoes (Ipomoea batatas). An alternative identification, 6-myoporol, was scored as 5.
-
Aspergillic acid - a mycotoxin produced by Aspergillus flavus.
-
Flavipucine.
-
Indole-3-acetic acid.
-
Muscimol.
Supermarket rearing substrate sample
One feature was scored as 9 (identity confirmed versus reference material data). This was identified as solanidine - an alkaloid produced by plants of the family Solanaceae (including potato, tomato, egg plant).
One feature was scored as 7 (strong indication of identity in the absence of reference standard). This was tentatively identified as galantamine - phytotoxin found in a number of Narcissus species, e.g daffodils. An alternative identification scored as 7 was pluviine, a phytotoxin often found alongside galantamine.
Five features were scored as 6 (indication of identity in the absence of reference standard). These were tentatively identified as:
-
Emodin.
-
Grayanotoxin III - a phytotoxin found in Rhododendron spp.
-
Grayanotoxin IV - a phytotoxin found in Rhododendron spp.
-
Menisdaurin - a phytotoxin found in Ilex aquifolium (common holly).
-
Rubellin D - a mycotoxin. An alternative identification, rugulosin, a mycotoxin, was also scored as 6.
Five additional features were scored as 5. These were tentatively identified as:
-
Chrysophanol - a hepatotoxic anthraquinone mycotoxin.
-
Metatyrosine - a phytotoxin found e.g. in Festuca arizonica (pine grass, native to North America) and Festuca rubra (red fescue, widespread in the Northern hemisphere).
-
Morphine - a morphinan alkaloid phytotoxin produced by Papaver somniferum (Opium poppy). Also used as a pharmaceutical and a drug of abuse.
-
Thebaine - a morphinan alkaloid phytotoxin produced by Papaver somniferum (Opium poppy, minor component) and Papaver bracteatum (Persian poppy, major component). Also used as a drug of abuse and as a starting point in the synthesis of other opioids.
-
Versiconol acetate - a mycotoxin.
Supermarket larvae sample
Two features were scored as 9 (identity confirmed versus reference material data). These were identified as:
-
Solanidine.
-
Alpha-chaconine.
One feature was scored 8 (strong indication of identity in the absence of reference standard). There were four possible related compounds (Figure 16) tentatively identified all of which are alkaloids produced by plants of the family Solanaceae (including potato, tomato, egg plant). There was no clear evidence as to which compound corresponded to which peak:
-
ß1-solanine.
-
ß2-solanine.
-
ß1-chaconine.
-
ß2-chaconine.
Four additional features were scored as 5. These were tentatively identified as:
-
Muscimol.
-
Penigequinolone A - a mycotoxin produced by Pencillium spp.
-
Scopine - a tropane alkaloid phytotoxin produced by a number of plant species including members of the genus Madragora and Scopolia carniolica (Henbane bell).
-
Trypyoquivaline E - a mycotoxin. An alternative identification, tryptoquivaline H, was also scored as 5.
Supermarket frass sample
One feature was scored as 8 (strong indication of identity in the absence of reference standard). There are four possible related compounds tentatively identified (Figure 16), with no clear evidence as to which compound corresponds to which peak:
-
ß1-solanine.
-
ß2-solanine
-
ß1-chaconine.
-
ß2-chaconine.
One feature was scored as 7 (strong indication of identity in the absence of reference standard). This was tentatively identified as convalloside - a cardiac glycoside phytotoxin, found in Convallaria majalis (lily of the valley).
Five features were scored as 6 (indication of identity in the absence of reference standard). These were tentatively identified as:
-
Emodin.
-
Indole-3-acetic acid.
-
Rubellin D - a mycotoxin. An alternative identification, rugulosin, a mycotoxin, was also scored as 6.
-
Tropine - a tropane alkaloid found in Atropa belladonna (deadly nightshade) and Datura stramonium (devil’s trumpet).
-
Tryptophol - classified both as a mycotoxin and a phytotoxin. Produced by the fungus Candida albicans and also found in Pinus sylvestris (Scots pine).
Nine additional features were scored as 5. These were tentatively identified as:
-
Anisomycin - a bacterial toxin with antibiotic action produced by Streptomyces griseolus.
-
Aristolochic acid I - a member of a group of phytotoxins (carcinogenic, mutagenic and nephrotoxic) commonly found in plants of the family Aristolochiaceae (birthworts).
-
Chanoclavine - an ergot alkaloid mycotoxin. Alternative identifications are the ergot alkaloids isochanoclavine, dihydroelymovlavine, dihydroisolysergol, dihydrolysergol and fumigaclavine B and the roquefortine alkaloid roquefortine B.
-
Chlamydosporol - a mycotoxin.
-
Cladosporin - a mycotoxin. An alternative identification is the mycotoxin curvularin.
-
Coumarin - a phytotoxin found naturally in many plants such as strawberries, tonka bean, cinnamon etc.
-
Domoic acid - a fycotoxin that accumulates in shellfish. A causative agent of amnesiac shellfish poisoning (ASP).
-
Homolycorine - a phytotoxin found in plants of the genus Narcissus (e.g. daffodil).
-
Tenuazonic acid - a mycotoxin.
Poultry manure rearing substrate sample
Three features were scored as 6 (indication of identity in the absence of reference standard) These were tentatively identified as:
-
Emodin.
-
Lupinine - a phytotoxin (hepato- and neurotoxin) found in plants of the genus Lupinus (Lupins).
-
Onchidal - a neurotoxic shellfish toxin acting as an irreversible acetylcholinesterase inhibitor, produced by species of the genus Onchidella (sea slugs).
Eleven additional features were scored as 5. These were tentatively identified as:
-
Dinophysistoxin-2 - a fycotoxin (algal toxin that accumulates in shellfish), a causative agent of diarrhetic shellfish poisoning. A related toxin, okadaic acid, is an alternative identification.
-
Chrysophanol - a hepatotoxic anthraquinone mycotoxin.
-
Conhydrine - an alkaloid phytotoxin found in Conium maculatum (poison hemlock, native to Europe and North Africa).
-
Cucurbitacin D - a phytotoxin found in Cucurbitaceae spp. (e.g. squash, courgette, cucumber, watermelon).
-
Cycloaspeptide F - a cyclic peptide mycotoxin.
-
Digitoxin - a cardiac glycoside phytotoxin found in Digitalis purpurea (foxglove).
-
Hebevinoside VI - a mycotoxin.
-
Hebevinoside X - a mycotoxin related to the previous compound.
-
Ipomeanine - a myco/phytotoxin, found in mould-damaged sweet potatoes (Ipomoea batatas).
-
Monocerin - a mycotoxin.
-
Versicolorin A - a mycotoxin.
Poultry manure larvae sample
One feature was scored as 5. This was tentatively identified as:
- Emodin.
Poultry manure frass sample
Four features were scored as 6 (indication of identity in the absence of reference standard). These were tentatively identified as:
-
Emodin.
-
Enniatin B - a cyclic peptide mycotoxin.
-
Enniatin B1 - a cyclic peptide mycotoxin related to the previous compound. An alternative identification is enniatin B4.
-
Scopine - a tropane alkaloid phytotoxin, produced by a number of plant species including members of the genus Madragora, and Scopolia carniolica (Henbane bell). Alternative identifications, scored as 5, are retronecine (a pyrrolizidine alkaloid phytotoxin) and scopoline (related to as an isomer of scopine).
Eleven additional features were scored as 5. These were tentatively identified as:
-
Chrysophanol - a hepatotoxic anthraquinone mycotoxin.
-
Citrinin - a mycotoxin produced by a number of fungal species e.g. Penicillium, Aspergillus spp.
-
Cucurbitacin A - a phytotoxin found in Cucurbitaceae spp (e.g. squash, courgette, cucumber, watermelon).
-
Cucurbitacin D - a phytotoxin found in Cucurbitaceae spp (e.g. squash, courgette, cucumber, watermelon). An alternative identification is cucurbitacin L.
-
Cycloaspeptide F - a cyclic peptide mycotoxin.
-
Desferrioxamine E - a bacterial toxin produced by Streptomyces pilosus.
-
Enniatin A1 - a cyclic peptide mycotoxin. An alternative identification is Enniatin G.
-
Hebevinoside III - a mycotoxin.
-
Hebevinoside VI - a mycotoxin, related to the previous compound.
-
Heliotrine - a pyrrolizidine alkaloid phytotoxin found in plants of the genus Heliotropium (wide-ranging and also used as garden plants).
-
Lupinine.
Currently non-permitted rearing substrates: Results of note
It is noted that very few compounds were scored as 8 or 9 and therefore for which the identification can be reasonably assured. The compounds scored as 9 were identified as solanidine and alpha- chaconine.
As discussed under the results for the currently permitted substrates there are currently no maximum specified levels for these glycoalkaloids in animal feed, but it is considered that further data on the presence of these compounds in animal feed is required before an evaluation can be made.
Although most of the compounds were scored between 5 and 7 and therefore the tentative identifications may not provide correspond to the actual compound present, the results may provide a guide as to the type of compound that may be present. The majority of compounds found were tentatively identified as mycotoxins, particularly in the frass samples. Further analysis with standards would be needed to ascertain the identity of these compounds.
Generally, there were fewer compounds identified in the larvae than in the rearing substrate or frass.
Microbiological analysis
Commission Regulation (EU) No 142/2011 specifies that feed materials should comply with the following limits for microbiological contaminants during or upon withdrawal from storage:
-
Salmonella spp: absence in 25 g n = 5; c = 0; m = 0; M = 0
-
Enterobacteriaceae: n = 5; c = 2; m = 10; M = 300 in 1 g
Where:
n = number of samples to be tested,
c = number of samples where the number of bacteria expressed in colony forming units (CFU) is between m and M,
m = threshold value for the number of bacteria expressed in CFU that is considered satisfactory,
M = maximum value of the number of bacteria expressed in CFU.
Salmonella, Campylobacter and ESBL E. coli were not detected in any extracts from any samples. Staphylococci were reported as <10 cfu/g for supermarket surplus, larvae reared on supermarket surplus and the frass produced. Levels of Staphylococci in kitchen catering waste were high but did not vary considerably between the rearing substrate, the larvae and the frass (means of 7.715, 7.288 and 8.224 log10 cfu/g respectively).
The level of Enterobacteriaceae specified in Commission Regulation (EU) No 142/2011 was exceeded in larvae from all rearing substrates except the core diet and core diet +20%. Larvae reared on currently permitted substrates were culled by blanching in boiling water (approx. 100°C) and cooking until core temperature exceeded 75°C. The larvae reared on the currently non-permitted substrates were killed by blanching in water above 80°C for three minutes. This method was used to kill the larvae but not to serve as a method to reduce the microbiological load to the specified limits. There are different processing methods that are used to manufacture processed animal protein from insect larvae and these include different protocols to kill the larvae together with subsequent processing to produce insect protein. It is highly likely that these processing methods will reduce the microbiological load significantly. Therefore, in the context of this project these results are likely to represent a maximum microbiological load that would be reduced by further processing.
It should be noted that larvae produced from the core diet and core diet + 20% had been processed in accordance with the commercial providers standard procedures to ensure regulatory compliance.
Commission Regulation (EU) No 142/2011 specifies that processed manure taken during or immediately after processing should comply with the following limits for microbiological contaminants:
-
Salmonella spp: absence in 25 g n = 5; c = 0; m = 0; M = 0
-
E. coli or Enterococcaceae: n = 5; c = 5; m = 0; M = 1000 in 1 g
Where:
n = number of samples to be tested,
c = number of samples where the number of bacteria expressed in colony forming units (CFU) is between m and M,
m = threshold value for the number of bacteria expressed in CFU that is considered satisfactory,
M = maximum value of the number of bacteria expressed in CFU.
EU regulation 2019/1009 also specifies the same limits as those given above for pathogens in an organic fertiliser. The maximum value of 1000 CFU/g was exceeded for Enterococcaceae in frass samples from all rearing substrates but was only exceeded for E. coli in frass from catering waste. These results indicate that further processing of the frass would be required to conform to regulatory requirements.
Individual results are shown in Figure 17 and full results are shown in Appendix I.
Non-targeted virus screen
Difficulty was experienced in extracting good quality RNA from the rearing substrate samples even after repeat extraction. A total of 848,589,172 read pairs passed the QC process with an average of 42 million read pairs per sample. The reads were assessed for duplicates prior to the normalised reads being assembled. Extracted RNA quantities, read numbers and normalised reads are presented in Table 8. As can be seen lower levels of RNA were extracted from the rearing substrate samples leading to lower levels of reads or lower diversity (low normalised reads percentages) in the resulting sequence data.
After QC trimming the resulting high-quality reads were assembled and compared to the GenBank NT database using BLASTN (Benson et al., 2015). Table 9 details all the viruses identified. They can be broadly classified into three groups based on their likely origin.
-
Viruses associated with bacteria, fungi or other organisms (excluding plant and avian) associated with the rearing substrate or the insect rearing process.
-
Plant viruses associated with the rearing substrate.
-
Avian viruses associated with the rearing substrate.
A range of plant pathogenic viruses were detected in the plant based feed and associated larvae and frass. More were detected in the larvae and frass than in the original feed. A range of chicken pathogenic viruses were detected in the poultry manure with some also passing on into the larvae and frass.
The aim of this study was to assess the likelihood of pathogenic viruses passing through the BSF production system if introduced from the original feed stock. The areas of concern are human and livestock viruses passing into the insect protein samples which are destined for consumption and plant and human viruses passing into the frass which may be used as fertiliser and spread on fields. The presence of viruses was assessed based on the presence of viral RNA detected by sequencing. This is the only method available which can assess the wide range of potential viruses which might be present, but it should be noted that presence of viral RNA in a sample although indicative of the original presence of a virus does not necessarily equate to the presence of an infectious virus in a particular sample.
The viruses detected were split into groups based on their likely origin. No human or non-avian livestock viruses were detected, which is not an unexpected result as the rearing substrates were from food or food grade manufacturing surplus or poultry manure and although the potential for such viruses exists it would be expected to be at very low level. In the absence of such viruses, it is difficult to assess the potential for their transmission though the system but by looking at the presence of viruses from similar classes some relevant information can be inferred.
Regarding plant virus presence, it is noticeable that more viruses were detected in the frass than in the original rearing substrate. A reason for this may be due to the diverse nature of the rearing substrates resulting in difficulty extracting RNA from these samples. This led to fewer reads and lower diversity of reads, which is likely to have reduced the detection efficiency for viruses (Pecman et al., 2018). Due to the diverse nature of the rearing substrates, 2.5 g samples of this matrix may be insufficient to be a true representation of viruses present in the whole sample. The frass is also likely to have been made more homogenous by the activity of the BSF larvae during development so the frass samples could be more representative of the diversity of the original sample. The frass and larvae particularly the catering surplus and core diet samples contain RNA from a range of common viruses known to infect vegetables such as tomatoes, potatoes, carrot, and lettuce. Significantly RNA from these viruses was present in the frass and larvae samples.
Based on studies of viral survival from disinfection (Noble et al., 2009) and composting experiments (Kerins et al., 2018) many of the viruses detected are unlikely to be viable after passing through the BSF digestive system or after pasteurisation of the frass. Others are transmitted via insects while feeding. This is unlikely / impossible from leaf debris in frass / larvae. This leaves only two organisms of concern. Apple hammerhead viroid which is not currently present in the UK and particularly Tomato brown rugose fruit virus. This virus is very stable (Skelton et al., 2023) and of ongoing concern for Defra and the UK tomato industry. Tomato brown rugose fruit virus was detected in the catering, supermarket and core diet samples. The detection of Tomato brown rugose fruit virus was not unexpected and is in agreement with recent work done at Fera on imported tomatoes (publication in preparation), which frequently found the virus on supermarket bought tomatoes and results from a similar study done in the USA (Yilmaz & Batuman, 2023).
Considering the avian viruses, the poultry manure is likely to have been much better homogenised by the chickens than the food waste. A diverse range of viruses including Infectious bronchitis virus, Rota viruses, enteric parvovirus, Megrivirus, Gallivirus and Avian nephritis virus were found in the rearing substrate but less seems to have carried through to the larvae with only Megrivirus still detected. More viruses were detectable in the frass with RNA from Infectious bronchitis virus, Megrivirus, Gallivirus, Anativirus, Sicinivirus and Avian nephritis virus present. As with the plant viruses the presence of RNA does not confirm the presence of infectious viral particles. The authors are not experts in avian viruses but from a literature search the viruses detected appear to be common in UK poultry and do not appear to be a risk to humans or other livestock.
The viral sequences detected in the frass and larvae come from a wide range of single and double stranded RNA based viruses and include at least one enveloped virus and one viroid. This suggests that RNA from many viruses could survive the BSF rearing process. What this screening has not shown is whether any of these viruses remain viable and are likely to be a risk to humans, animals or plants. Infection studies would need to be carried out to determine if this is a risk. With the high levels detected, known stability and threat to the tomato industry, Tomato brown rugose fruit virus would seem an ideal candidate for such infection studies.
In summary, RNA from a range of avian and plant viruses was detected in the frass and larvae of BSF. It is not known if any of these viruses were still infectious but if they were they may pose a risk to animal and plant health and by inference open the possibility that if human viruses had been present, they also might have been a risk.
Assessment of the extent to which the results are representative of the substrate, larvae and frass.
A priority for assessment of chemical and microbiological safety is ensuring that experimental findings for each rearing substrate are, as far as possible, representative of the long run average of the real-world systems for which they are models. Achieving this rests on the analysis of representative samples. Representativeness means that if the long-run average of the concentration of a hazard in the real-world system is above the limit of detection, or other critical concentration then we should detect its presence in this study. It also means that for a particular hazard in a substrate, the probability that a detectable quantity is presented for analysis should depend only on the size of the sample presented for analysis and the long-run mean quantity of the hazard (definition adapted from “Object of sampling” ISTA, 2022).
In addition to gaining a measure of the mean hazard, a more completely representative assessment would include an estimation of the size of the variation in the concentration of hazards between manufacturers, batches of material and at smaller scales. This is particularly relevant for potential acute hazards. However, this would entail the analysis of a large number of analytical samples at great cost. Hence, the aim of the current assessment was to provide information about the mean presence of hazards that is robust to the variation, rather than to describe how variable individual instances of the material may be. The goal of the sampling designs used in this project was to deploy limited resources (sample handling capacity; number of analytical tests) as efficiently as possible to provide a reliable estimate of the mean presence of hazards and, in particular, to ensure that any localised risks (e.g. those associated with only a subset of the material in a particular substrate group) have a sufficient chance of being detected (Lundy & Parrella, 2015; Varelas, 2019) with sufficient replicate samples and testing to provide assurance that the mean estimates are robust.
Analyses were undertaken with the aim of determining the average concentration of substances and microbiological content in substrate, larvae and frass for each of catering, manufacturing and supermarket surplus and poultry manure. An A sample and B sample were formed from independent sets of primary samples. Two separate sub-samples were taken from the B sample. Samples were analysed in duplicate. (For PFAS, toxin screen and non-targeted viral screen all samples were combined into a C sample which was analysed once).
The average concentration is estimated as
Estimate =(A+((B1+B2))÷2
Hence, if the standard deviation of between-sample variation (A, B) is and the standard deviation of duplicates taken from an independent sample is then the standard error of the estimated average for the type and source is:
se=√((σ−B∧2)/2+(3.σ−W∧2)/8)
We can say that an upper limit for a value of se for a useful measurement result which provides evidence that an average concentration is above a limit of assurance that an average concentration is below a limit is:
se ≤∣limit −estimate∣÷2
The critical difference (p=0.05) for the difference between the mean of B1, B2 and A (i.e. the observed difference between independent samples) is given by:
〖diff〗_95≤2√(2.σ−B∧2+(3.σ−W∧2)/2)
We were unable to undertake enough replicate analyses to estimate values of and within the budget of the project. Hence, we are limited to identifying cases where the variation between results appears to be large compared with the variation we need to be confident about whether a mean is above or below a limit. This is because the results produced by only two independent sample may be close to each other by chance even when the underlying variation is large.
The variation we observe between samples has two sources:
-
Between-sample variation: this is variation in the concentration of analyte between samples despite our efforts to create samples that contained the analyte at the mean concentration in the bulk.
-
Analytical variation: the variation we might see between measurement results even where the concentration in samples is the same.
We expect that analytical variation for chemicals will be no higher than approximately 20% relative standard deviation, based on the Modified Horwitz Relative standard deviation of 22% for analytes at very low concentrations (Thompson, 2000). We also expect that the analytical variation for microbiological analytes will be no higher than a standard deviation of 0.5 Log10 units for microbiological analytes (Public Health England). Hence, where between-sample variation is no larger than analytical variation we expect that for the particular patterns of replication that we employed that for chemical analytes, the difference between replicate samples (divided by the mean result) to be no larger than 0.58 (95% confidence, estimated by simulation). For microbiological counts we expect the difference between duplicate samples to be less than 1.44 Log10 units. Table 10 shows instances where the difference is exceeded.
The majority of between replicate variation was found for the rearing substrate. As previously hypothesised, this is likely to be due to the rearing substrate being less homogenous than the larvae and the frass. It is therefore recommended that when assessing contaminants in rearing substrate a greater number of samples are assessed.
Conclusions and recommendations for further studies.
This study has evaluated the chemical and microbiological contaminants in currently non-permitted rearing substrates for BSF larvae for production of protein for animal feed, the larvae produced and the remaining residue from the process (frass). The materials tested represented four different categories of substrate that are suitable for rearing of BSF. However, only a single source from each category was evaluated in this study and the samples obtained were taken over a short time period. This should be kept in mind when assessing the results.
Analytical methods screened for 745 chemical analytes, the presence and enumeration of key microbial organisms was assessed, and non-targeted screens were used to assess the presence of natural toxins and viral RNA that were present in the samples.
A summary of the number of chemical compounds found in the different sample types is provided in Tables 11-13. It should be noted that the same compound is not necessarily present in the rearing substrate, the larvae and the frass.
Of the chemical analytes screened for a total of 101 were found in the larvae. The majority of these were metals (58). Of the analytes found, there were no exceedances for any of the chemicals where maximum limits are specified in feed materials of animal origin. However, some pesticide residues exceeded the MRLs for terrestrial invertebrates. This may have implications if the larvae were to be used as food.
The only regulatory limit exceeded for feed ingredients of animal origin, was for the presence of Enterobacteriaceae in larvae reared on all four of the currently non-permitted substrates. As previously discussed, this was likely to be due to the minimal processing used to kill the larvae. The regulatory levels specified refer to samples taken after the application of a processing method and therefore the results from this study illustrate that processing is required to reduce the microbial load. It is likely that processing methods typically used by industry for the production of insect protein would significantly reduce the level of these organisms, as demonstrated by the results from the baseline samples. However, this would need to be confirmed and supported by HACCP and GMP procedures.
Although the levels of contaminants were below regulatory limits for feeding stuffs of animal origin, there was evidence of bioaccumulation in the larvae for some compounds. Cadmium was shown to bioaccumulate in BSF larvae as previously reported. For metals, there was also evidence of bioaccumulation of lead, calcium, phosphorus and some other metals. Although this may not have a direct safety concern, this may have implications (positive or negative) for some metals that serve as macro- or micronutrients. There was also evidence of bioaccumulation of DDAC and haloxyfop in larvae reared on poultry manure. Other contaminants such as mycotoxins and PAHs also gave indication for potential bioaccumulation but variation between samples requires that further testing would be needed to ascertain whether this is the case.
As far as the authors are aware this is the first study that has examined the presence of PFAS through the insect bioconversion process using naturally occurring levels of these compounds. Some compounds were found in the rearing substrate, the larvae and the frass, but a more extensive study is recommended to confirm these findings. Further optimisation of the method is required to analyse the diverse range of substrates that may be used as a rearing substrate for insect bioconversion.
Commission Regulation (EU) No 142/2011 specifies that processed manure taken during or immediately after processing should comply with limits Salmonella spp. and Escherichia coli or Enterococcaceae. The maximum value of 1000 cfu/g was exceeded for Enterococcaceae in frass samples from all rearing substrates but was only exceeded for E. coli in frass from catering waste. These results indicate that further processing of the frass from currently permitted and non-permitted substrates is required to conform to regulatory requirements.
The non-targeted viral screen confirmed the presence of RNA from plant and animal pathogens in larvae and frass, but the infectivity of these viruses could not be elucidated from this study. Infection studies should be carried out to determine if this is a risk, particularly for plant pathogens when frass is used as a fertiliser.
The non-targeted toxin screen demonstrated the presence of natural toxins such as solanidine in larvae. Currently there are no regulatory limits for the presence in animal feed and an EFSA risk assessment concluded that a risk characterisation of potato glycoalkaloids in feed for farm and companion animals was not possible due to insufficient data on potential adverse effects in these species (EFSA, 2020). Data on the presence of these compounds in a range of animal feeds is therefore required before an assessment of the levels found in insect larvae can be evaluated. The non-targeted toxin screen also tentatively identified other natural toxins in larvae reared on currently permitted and non-permitted substrates, but scores for these were generally low (5-7) and further work would be needed to confirm the identity of these compounds.
It was noted that several compounds were not detected in the rearing substrate but were detected in the larvae and/or the frass. It is considered that this may be due to the greater homogeneity of the larvae and the frass samples compared with the rearing substrate such that there may have been a more even distribution of any contaminant present.
Examination of the variation in results between replicates showed that the majority of between replicate variation was found for the rearing substrate. This is likely to be due to the rearing substrate being less homogenous than the larvae and the frass. It is recommended that when assessing contaminants in rearing substrate a greater number of samples are assessed.




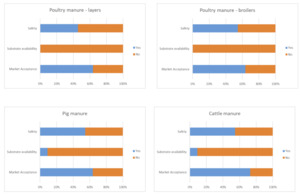


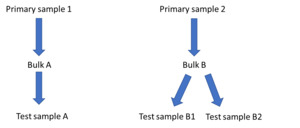



__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)





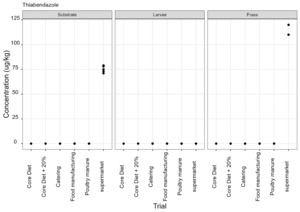
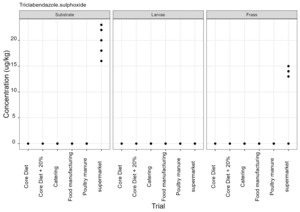


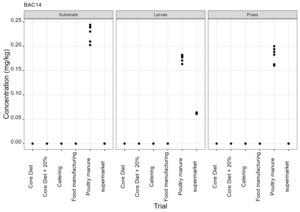







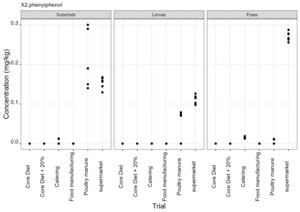




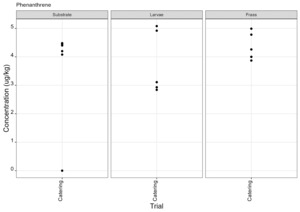

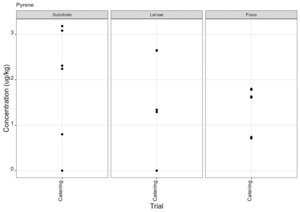
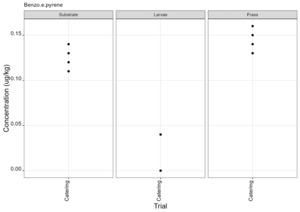







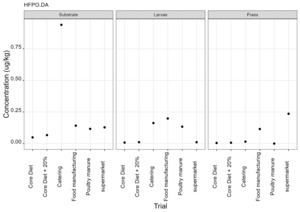

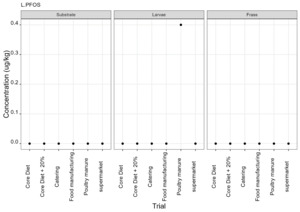

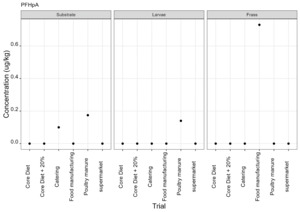

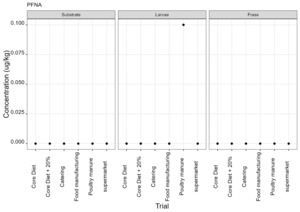
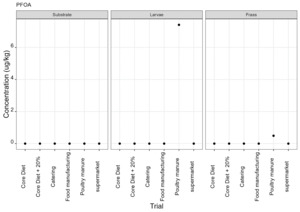

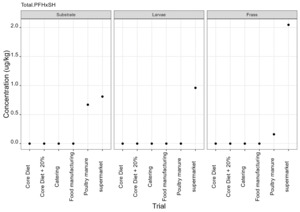


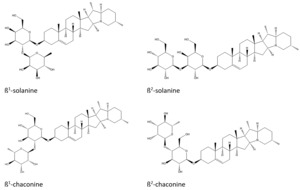



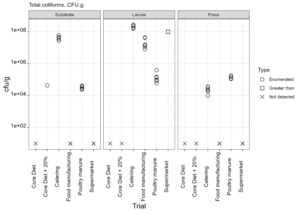

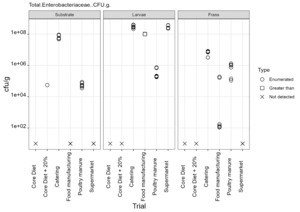
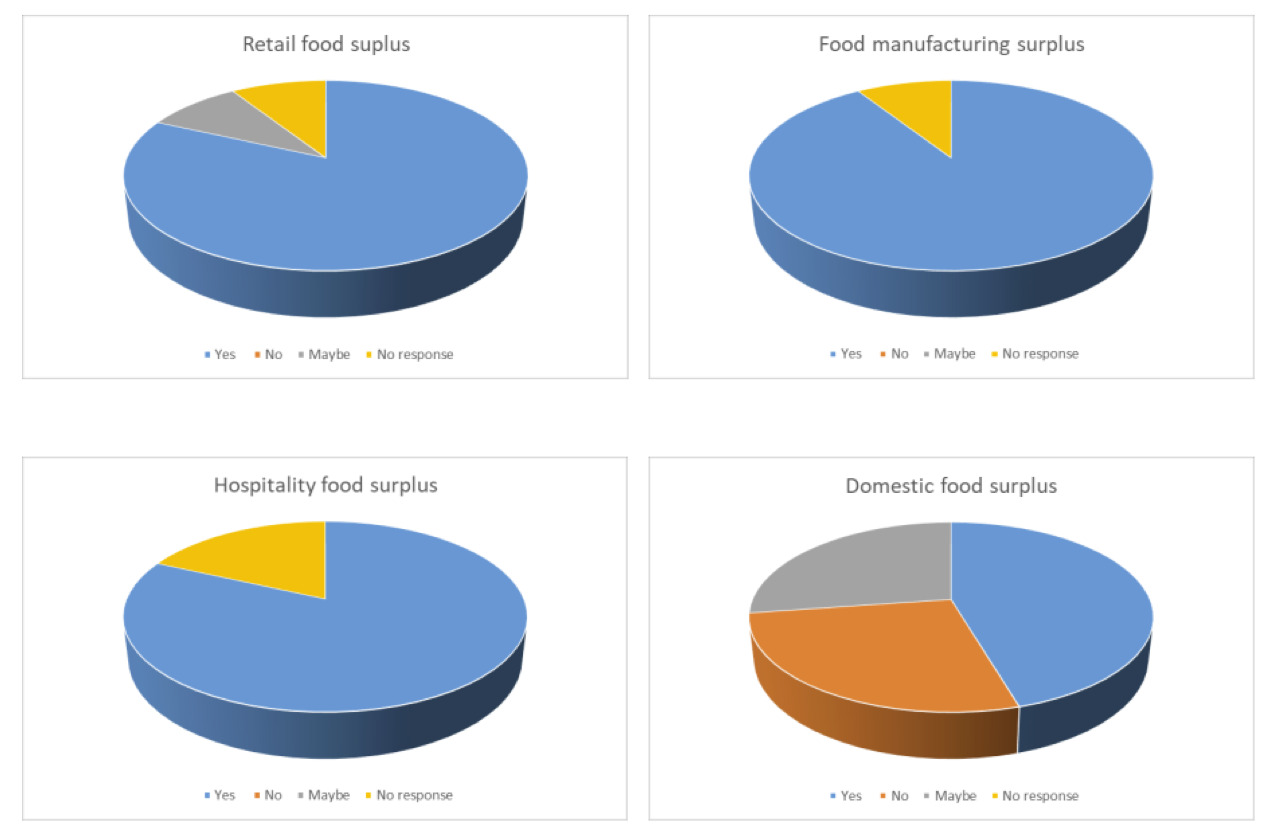
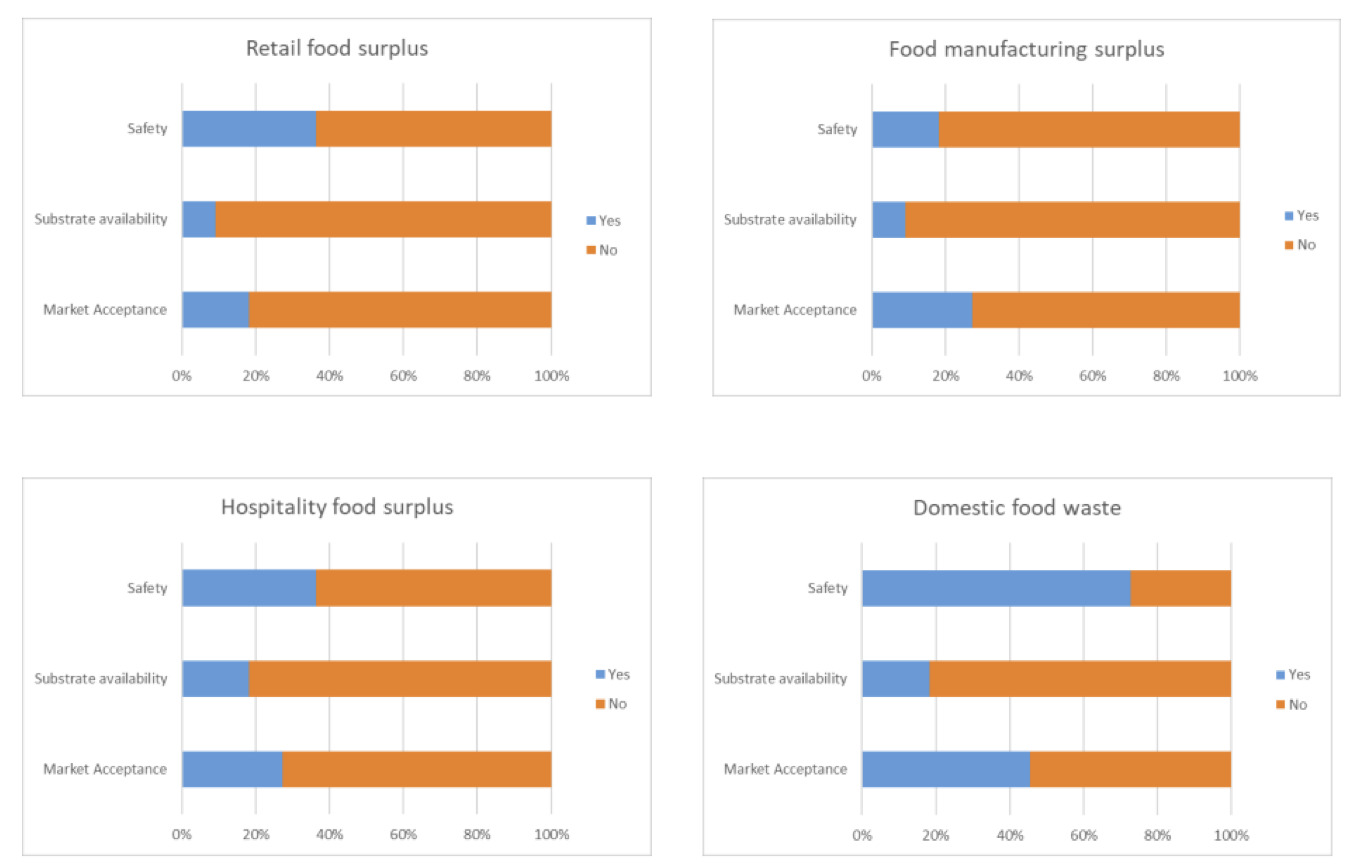


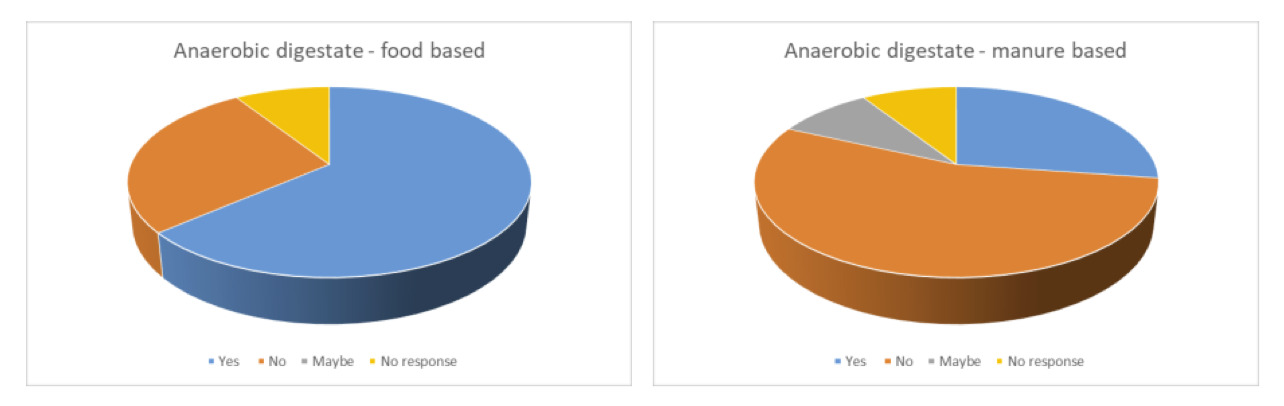





__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)
__cadmium_(b)__mercury_(c)__and_lead_(d)_found_in_different_.png)