1. Introduction
FSA and FSS have undertaken safety assessments for isolated Cannabidiol (CBD) derived from hemp (Cannabis sativa) under the novel foods legislation, assimilated Regulation (EU) 2015/2283. To support the safety assessment, the ACNFP provided the advice outlined in this opinion to the FSA and FSS.
The evaluation assessed the food safety risks of the novel food and its production, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Authority (EFSA) for full novel food applications is used as the basis and structure for the assessment (EFSA NDA Panel, 2016).
An application was submitted to the Food Standards Agency (FSA) and Food Standards Scotland (FSS) in January 2021 from EIHA Projects GmbH. (“the applicant”) for the authorisation of isolated CBD as a novel food. The novel food is a >98% pure form CBD which is intended to be used as a food supplement for adults.
Advice was sought from the joint Subgroup of the ACNFP and the Committee on Toxicity (COT) on CBD and hemp derived products, on the quality of the toxicological evidence submitted to support the application. The ACNFP and COT have issued a joint statement on a provisional ADI that can be applied to CBD ingredients of >98% purity. This, and wider evidence available in the public domain, was taken into account in reviewing the toxicological evidence for this application.
The final advice from the Committee was agreed at the 164th meeting, allowing the FSA and FSS to complete the risk assessment.
The document outlines the conclusions of the FSA and FSS on the safety of a >98% pure form CBD Isolate (as detailed in application RP427) as a novel food and represents the opinions of the FSA and FSS.
2. Assessment
2.1. Identity
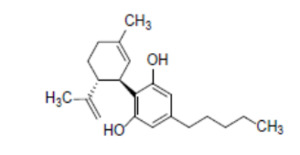
The novel food is a hemp-derived CBD isolate of ≥98% purity, presented as a colourless-to-yellow crystalline powder. This is dissolved and standardised to between 2.5% and 10% CBD concentration in hemp seed oil. Representative information to support this characterisation was provided by four independent EIHA members for five batches of the novel food.
CBD is characterised by the chemical formula: C21H30O2; molecular mass: 314.46 g/mol; CAS number: 13956-29-1; IUPAC name: 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol.
Confirmation of its identity was provided by IR spectroscopy, HPLC-DAD, HPLC MS-MS, and LC/MS-MS.
2.2. Production Process
Details of the production process were provided by the applicant and reviewed by the ACNFP. The basic steps of the full production process are as follows: Cultivation and harvesting of raw hemp; drying of the raw hemp; production of primary extract through solvent extraction; decarboxylation; winterisation; distillation; isolation of CBD through solvent extraction; crystallisation; filtering, washing, and drying; final formulation and dilution in hemp seed oil.
Not all EIHA associated partners take part in all steps of the production process and are categorised by the applicant as either “primary producers” or “secondary producers”. This is due to members purchasing raw hemp biomass, or CBD isolate, to formulate their own products without participating in upstream processes. Therefore “primary producers” are defined as those producing both hemp and CBD isolate; whereas “secondary producers” are defined as those who purchase any downstream product to use as an ingredient in their own formulations. Each novel food pertinent to this application would still follow the overarching production process described and the novel food would be produced to the specification outlined in Section 2.5.
There may be some variation in production process between members of the EIHA. The applicant states that the process varies between members during growth of the hemp biomass and harvesting of the hemp biomass. This is due to the geographic and climatic differences affecting the growth and harvesting conditions required to meet the specifications. There are variations in the solvents used as part of extraction and purification of the CBD which result in different production processes. However, the specification set for the isolated CBD remains the same for all production processes and includes solvent residues for each of the processes used.
The potential hazards from the solvents used by partners are considered in the relevant section. Certificates of analysis for raw starting materials used in the production of both CBD isolate and CBD isolate in hemp seed oil were provided, to demonstrate the effectiveness of the controls at this point in the process. The data is provided for one partner as an example of the effectiveness of controls in the partners systems.
The source material used for the production of the CBD isolate is the flowers and leaves of the Cannabis sativa L. plant (also known as “hemp” or “industrial-hemp”). The specific varieties of hemp used vary depending on the individual associated product partner. However, all varieties used are included in the EU Plant Variety Database and comply with, or are lawfully grown within Europe, according to EU Regulations 1307/2013 and 1308/2013.
Based on the ACNFP’s advice the FSA and FSS considered whether the use of solvents as processing aids resulted in residues that require highlighting to risk managers. Four potential extraction methods are identified as being used by partners: ethanol extraction, CO2 extraction, 2-propanol extraction, or 1,1,2-Tetrafluoroethane extraction. The other solvents are used as part of the crystallization process. To assess the safety of the solvent residues that remain in the novel food, comparison was made to residue limits for other consumed products as detailed in table 1 - residues of solvents included in the specification.
The evidence presented on composition shows the residues of solvents in the product were consistently below the level set in the specification. When considered at the level of consumption the evidence suggests the levels of solvent residues in the novel food are below those which would represent a safety concern.
2.3. Compositional information
Results from five independent batches of the novel food, produced through each of the four production methods, are provided within Tables 2 – 5. These results demonstrate that the novel food can be produced consistently. The data presented in each table is from a different representative manufacturer implementing each of the extraction method described within the production process.
CBD content is consistently above 98% with negligible amounts of starting materials detected across the five representative batches.
It is recognised that the detection and characterisation of cannabinoids in a range of food matrices is an evolving area and there are yet to be internationally recognised methods. The limitations of analytical methodology available have been subject to discussion in the Joint ACNFP and COT CBD Subgroup and remain a source of uncertainty in the assessment. As a result, the robustness, accuracy, and precision of the methods have been considered in interpreting the data on THC and were considered appropriate in this case.
Cannabinoids other than CBD present at low levels that may be considered as a contaminant, have been considered as part of the application. In particular, delta-9-tetrahydrocannabinol (THC) is analysed due to the potential for toxic effects resulting from its consumption and its illegal status within the UK. Along with THC, other minor cannabinoids which occur at contaminant levels have the potential to play a role in the toxicity of CBD novel food products; as such, they require due consideration and monitoring to ensure the novel foods remain safe.
The analysis for delta-9-tetrahydrocannabidiol as a potential contaminant in the novel food detected the substance at trace levels in all five batches tested. This may be present as a result of interconversion between cannabinoids or through cross contamination of the source material.
A literature review was undertaken as part of the assessment of CBD as a novel food, to understand the impact on the safety of foods with trace levels of contamination with THC. The joint ACNFP and COT subgroup reviewed the information from literature and identified a point of departure from the European Food Safety Authority (EFSA) opinion on THC as a contaminant in milk and meat (EFSA, 2015).
Evidence from an EFSA review by the CONTAM panel suggests a point of departure from a LOAEL (Lowest Observed Adverse Effect Level) of 0.036 mg/kg/bw/day, which is drawn from the most sensitive individuals and at the lowest dose tested in the clinical studies that were reviewed. Uncertainty factors were then applied to identify a safe upper intake level. These included an uncertainty factor of 3 to extrapolate from a LOAEL to a NOAEL (No Observed Adverse Effect Level); which was considered appropriate considering the effects are mild to moderate in severity. A further factor of 10 was applied to account for individual variation, resulting in-total to an applied uncertainty factor of 30. This resulted in a safe upper intake level of 1 µg /kg bw/day for THC consumed as a contaminant in food.
The Subgroup agreed the Acute Reference Dose (ARfD) to be sufficiently protective to apply to the UK population. It was noted that in applying the acute reference dose EFSA has assumed that the effects seen would be the same if humans were exposed to multiple doses of THC at very low levels. The Subgroup commented that there was no data to verify this assumption, but if setting limits the dataset is the best available.
The levels of THC in the novel food, once adjusted to the proposed use of 10 mg of CBD being consumed a day, were below the ARfD identified by EFSA of 1 µg/kg bw/day or 70 µg/day for a healthy adult. This level does not present a concern in terms of consumer safety for the novel food under the proposed conditions of use.
To ensure THC levels remain consistently low in the production of CBD, THC should be a standard substance included in the specification as relevant to all batches produced.
It is expected that novel food products comply with the legal requirements for heavy metal contaminants in foods. Analytical data, presented as the mean of five independent batches of the novel food, demonstrated that heavy metals were present in very low levels and were below established UK regulatory limits where applicable (arsenic, cadmium, mercury, and lead).
Analytical data concerning the microbiological content from five independent batches of the novel food were reported. The process in manufacturing this novel food uses several alcohol-based solvents, high temperature steps, and drying, which may mitigate the proliferation of microbes within the final product. The microbiological data presented confirm that the novel food does not raise a safety concern and consistently meets the proposed microbial specification levels.
The data presented indicated the novel food can be produced consistently and did not indicate any additional hazards for inclusion in the specification.
2.4. Stability
The stability of five batches of the novel food, and five batches of the novel food in hemp oil, was assessed under accelerated conditions at 40°C for 36 weeks. Results showed there were no significant changes in cannabinoid content or microbiological profile over this period. Analysis for THC was included in the stability studies, which showed the level of THC remained consistent within the specification limits during the study.
The data provided supports the stability of the novel food for a period of 24 months.
2.5. Specification
The specification parameters reported in Table 6 were assessed using internationally recognised methods or determined using internally developed and validated methods. Due to the nature of the application, covering multiple production processes using different methods of extraction, the specifications have been provided for each. Data has been supplied by the applicant to demonstrate that the product is produced consistently to one of the three specifications outlined in Table 6.
The FSA and FSS concluded based on the advice of the ACNFP that the information provided is sufficient for the specification of the CBD isolate described in application RP427 and appropriately characterises the novel food seeking authorisation. The Committee made clear the importance of products complying with the specification in order to be deemed as safe. Any EIHA associate member products which are unable to meet the specification laid out in this document would not be deemed safe.
2.6. History of Use
Hemp has been widely consumed in the UK and EU as a seed oil, in tea and as an alternative to hops in beer. Extracts of hemp including CBD and synthetic CBD have not been widely consumed and are considered novel foods. While CBD products are widely available on the UK high street, indicating some consumption of CBD as a food, no applications for CBD have yet received authorisation as a novel food. Any products which are currently on the UK market therefore remain non-compliant with the novel food regulations.
As detailed in the COT review of the literature there has been use of both hemp derived and synthetic forms of CBD for medicinal purposes. These provide an indication of the toxicological effects that should be explored in the testing regime – primarily effects on liver, thyroid and potential impacts on reproductive organs. Also reported are behavioural effects such as somnolence (sleepiness).
As reported in the COT review of the publicly available data on CBD and summary data on a medicinal product, signs of adverse effects on the liver were observed at doses of CBD as low as 5 mg/kg bw/day in patients and healthy human volunteers; this dose is equivalent to 350 mg in a 70 kg adult. The data in the literature also suggested that humans might be more sensitive to the adverse effects of CBD in the liver than laboratory animals.
Somnolence effects were noted at doses ≤10 mg/kg bw/day in human studies. Inhibitory drug-drug interactions have also been observed with some medications when CBD is co-administered at doses of 1 mg/kg bw/day (equivalent to 70 mg in a 70 kg adult); the likelihood of effects at lower doses has not been determined.
Based on the COT assessment, therefore, the FSA concluded in February 2020 that 1 mg/kg bw/day, or 70 mg in a 70 kg adult, of CBD represented a pragmatic upper level of intake above which there would be clear concerns about safety, until further data are available. This position has been updated in light of data received to support applications for CBD as a novel food. Further details on this are provided in the toxicology section.
It is noted that the doses used for medicinal purposes are higher than those proposed for food use. The purpose of an assessment for medicines authorisation is different to that for food and this is reflected in the data requirements. Unlike medicines, there is no risk-benefit context in foods with the requirement instead being that the products are safe. This means that outcomes that are considered an adverse event for food might not be considered as such in a medicinal study.
Within the literature, further human studies utilising chemically derived CBD provides further evidence of a history of synthetic CBD use (Izegelov et al., 2010; Klotz et al., 2019; Stero Biotechs Ltd., 2020; Wheless et al., 2019). A review by Heuestis et al., 2019 of Cannabidiol Adverse effects and Toxicity notes that, while CBD is not risk-free, severe adverse events occur at doses higher than those recommended for human pharmacotherapies which are prescribed to treat forms of epilepsy. The data on previous consumption of CBD suggest areas for careful consideration in the toxicological review to understand potential effects at the lower doses used in foods.
2.7. Proposed Use and Anticipated Intake
The proposed use for the novel food is as a food supplement in the form of an oil sold as capsules and drops at the dose of 10 mg per day of CBD (Table 7). The intended use is food supplements as defined by UK legal requirements (The Food Supplements (England) Regulations 2003, No. 1387, England, 2003) as capsules, liquid or drops in dose form. A provisional acceptable daily intake (ADI) for the use of >98% pure form CBD has been established at 10 mg per day (October 2023) and discussed in the Toxicological information section.
Concerns were raised by the Committee and noted in the FSA and FSS assessment regarding any further uses there may be for the novel food. It must be noted that the reformulation of the novel food, or incorporation of the novel food into other food products, would not be permitted under this authorisation. This is because of the increased risk of consuming CBD above the recommended ADI due to the potential exposure from multiple sources. As such, the only permitted use of the novel food is within hempseed oil as a food supplement up to a maximum daily intake of 10 mg.
It is noted that there are already many products available containing CBD. As such, the assessment has been made on the basis of identification of a maximum level of CBD that can be consumed per day per product. As such proposed uses will only be considered safe for all consumers within the assessment when below an intake maximum of 10 mg of CBD per day.
Risk managers must consider whether consumers would benefit from information on the CBD content of foods in order to ensure their consumption does not exceed a provisional acceptable daily intake of 10 mg per day for a healthy 70kg adult.
As recommended in the ACNFP and COT statement on CBD of >98% purity, “The provisional ADI is recommended, subject to the existing advice to consumers that pregnant and breastfeeding women and people taking any prescription medication should avoid the consumption of CBD. Consumers on regular medications should seek advice from a medical professional before using any type of CBD food product. In addition, children and prospective parents trying for a baby are advised against consumption of CBD, as are those who are immunosuppressed, due to remaining data gaps and residual uncertainties concerning the safety of CBD for these groups of consumers.”
The food supplement products are to be labelled in accordance with the labelling requirements of Food Supplements (England) Regulations 2003 as follows: Does not exceed the safe limit of 10 mg/day for a 70 kg healthy adult. Not suitable for use under the age of 18. Not suitable for use during pregnancy or breastfeeding. If you are taking medication or have existing health conditions, please consult your doctor before using this product.
The assessment explored the potential for foreseeable misuse of the novel food. It is highlighted to risk managers that they may wish to consider whether risk management measures are needed beyond those in the food supplements regulation to ensure consumers are aware of the provisional ADI of 10 mg CBD/day for the product, a dose at which it is considered that no adverse effects would be expected.
2.8. Absorption, Distribution, Metabolism and Excretion (ADME)
The Absorption, Distribution, Metabolism and Excretion (ADME) of cannabidiol are known to be complicated by the food matrix in which the CBD is delivered and are currently still being defined by professional bodies.
The oral bioavailability of CBD is low, indicating that it is not absorbed to any notable extent following ingestion (Mechoulam et al., 2002). Published works report the bioavailability of CBD to be between 13 and 19% (Grotenhermen (2003)) or 6% (Hawksworth and McArdle (2004)). The low systemic availability was demonstrated by Martin-Santos et al., 2012 and further supported by a literature search which identified the pharmacokinetics of CBD (Millar et al., 2018) The COT statement on CBD of 2020 noted that although CBD has low fasting bioavailability (<10%), consumption with food could increase CBD uptake, by for example, 5 fold if eaten with a high fat meal. As such the potential for matrix effects that impact bioavailability cannot be ruled out.
Following oral consumption, CBD is extensively metabolised in the liver. This rapid first pass metabolism contributes to the low oral bioavailability reported in the literature (Taylor et al., 2018; WHO, 2018). In vitro studies indicate that CYP3A4 and CYP2C19 are the primary hepatic enzymes responsible for first-pass metabolism of CBD; however, several other hepatic cytochrome P450 isoforms (CYP1A1, CYP1A2, CYP2C9, CYP2D6, and CYP3A5) have also demonstrated a capability of metabolising CBD (Jiang et al., 2011; Zendulka et al., 2016).
The metabolism of CBD is thought to follow two separate pathways. One is P450-mediated, in which CBD is metabolised into its major metabolite 7-COOH-CBD (which is a chemically inactive compound). This is followed by further metabolic reactions which yield the minor metabolites of CBD, including 6-OH-CBD (Devinsky et al., 2018; Taylor et al., 2018). The other involves decarboxylation (Kraemer et al., 2019). The resultant metabolites are predominantly excreted in faeces and urine (Hawksworth and McArdle, 2004; WHO, 2018).
Multiple dosing with CBD is associated with a steady state concentration up to 2-fold accumulation of CBD in plasma when compared with a single dose (Taylor et al., 2018). Minimal evidence of plasma accumulation has also been reported in dosing studies over 5–9 days (Millar et al., 2018; Sellers et al., 2013; Stott et al., 2013).
The pharmacokinetics of CBD have been systematically reviewed by Millar et al., (2018) in 24 studies, most of which assessed the administration of CBD at doses of 5–20 mg/day. This correlates to a low dose application similar to this CBD novel food application. Following oral administration, single doses of 5.4 and 10 mg CBD achieved peak serum concentrations (Cmax) of 0.9 and 2.5 ng/ml. The time to maximum concentration (Tmax) was approximately 1 h, with a half-life between 1 and 3 hours. Given the intended use of this CBD as a food supplement, with an approximate half-life of one to three hours, with a total clearance of six hours, there are no significant concerns of accumulation.
The ADME data provides context for interpreting the toxicological data. It is noted that the bioavailability of CBD is typically low but can be affected by food matrix. The food context for the novel ingredient could impact on CBD bioavailability. It was noted that the potential for CBD to accumulate in the body has not been examined based on the data supplied. This has been taken into account in considering the assessment factors to account for uncertainty in setting the provisional ADI.
2.9. Nutritional information
The data on nutritional composition confirms that CBD has no caloric or nutritional value. The application is not intending that CBD replace another food in the diet. Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
Clarification was sought on the potential for the presence of antinutritional factors from the preparation. It was noted that hemp can contain a range of substance that could impact the digestion and absorption of nutrients from the diet. These include phytic acid (which can negatively affect the bioavailability of dietary and endogenous minerals and proteins), tannins (which can interrupt the absorption of iron), trypsin Inhibitors (which can affect protein digestion), and saponins (which at larger quantities cause gastric irritation and increase the permeability of the intestine).
The product is highly purified as indicated in the information on the composition. There is no presence of other components that would impact the digestion or absorption of nutrients from the diet.
2.10. Toxicological information
Toxicological studies were performed with a cannabidiol isolate of 98% purity to support the safety assessment of the novel food. The respective study reports are unpublished and claimed as confidential and proprietary data. They were considered essential in the assessment the safety of the novel food. How data on systemic toxicity was managed and interpreted in the context of the provisional ADI is explained in the subchronic toxicology section below.
2.10.1. Genotoxicity
In vitro genotoxicity testing of cannabidiol was conducted under Good Laboratory Practice (GLP) conditions and utilised the followed OECD guidelines: in vitro bacterial reverse mutation test (Eurofins Munich Study No.: STUGC21AA0231-2) and in vitro mammalian cell micronucleus test (Eurofins Munich Study No.: STUGC21AA0231-3). This approach is recommended by the UK Committee on Mutagenicity and is also the basis of guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283, as assimilated into UK law.
The in vitro bacterial reverse mutation test demonstrated that cannabidiol was non-mutagenic in the absence and presence of metabolic activation. In addition, the in vitro mammalian cell micronucleus test demonstrated that cannabidiol was non-clastogenic and non-aneugenic in the absence and presence of metabolic activation.
The results from these in vitro studies support the conclusion that the novel food is not genotoxic. This is consistent with the view of the Committee on Mutagenicity in reviewing CBD as a substance (Committee on Mutagenicity; MUT/MIN/2020/1, 2020).
2.10.2. Sub-chronic toxicological study
The joint subgroup of the ACNFP and COT was formed to address a series of questions in relation to the safety of CBD, cannabinoids, and hemp-derived ingredients.
A weight of evidence approach has allowed the Subgroup to identify a provisional ADI for CBD ingredients, of >98% purity, of 0.15 mg/kg bw/day or 10 mg per day for a 70 kg healthy adult (Joint position paper from the ACNFP and COT; FSA consumer advice published in October 2023). This value was identified to be protective of the most sensitive known effects in the liver and thyroid parameters and included consideration of data gaps and uncertainties.
2.10.3. Subchronic data presented to support this application
This applicant provided a repeated dose 90-day oral toxicity study in rodents, [(BSL Munich Study No.: 2100110)] which was conducted under GLP conditions and OECD TG 408 guidelines. In this 90-day feeding study, each group comprised 10 female and 10 male rats which were dosed with 0 (control – corn oil), 25, 50, 75, or 150 mg/kg bw/day cannabidiol once per day by oral gavage at a dose volume of 4 mL/kg bw/day.
Review of the study supported the conclusion that it was of sufficient quality to support the safety of the novel food. The findings of the study were consistent with those considered in the development of the provisional ADI. It was, therefore, considered scientifically appropriate to apply the provisional ADI of 0.15 mg/kg bw/day or 10 mg/day as identified in the joint statement of the ACNFP and COT on >98% pure forms of CBD.
2.11. Allergenicity
This CBD isolate comprises >98% CBD and the production process for CBD does not introduce any risk of allergenic potential. CBD as a chemical entity suggests the potential for IgE mediated food allergy is unlikely.
None of the raw materials or processing aids used in the production process are derived from or contain any of the allergenic food ingredients specified under Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Suggesting the potential to elicit reactions in those sensitive to those foods is unlikely.
The novel food is unlikely to trigger allergic reactions in the target population under the proposed conditions of use.
2.12. Discussion
The novel food is an isolated cannabidiol-containing product containing ≥98% CBD, produced using a multi-step manufacturing process from raw hemp biomass.
This CBD is intended to be used as an ingredient in food supplements for adults at a dose of 10 mg a day; it is not intended to replace any food. A safety concern was raised regarding the potential future uses of the novel food as an ingredient in other foods. Further uses are not specified within this application and would therefore not be permitted under this authorisation.
In October 2023, the Joint ACNFP and COT subgroup identified a provisional acceptable daily intake (ADI) of 10 mg per day (0.15 mg/kg bw/day) for CBD novel foods containing 98% CBD or above, such as the novel food discussed in this assessment. A weight of evidence approach was used to arrive at a provisional ADI of 10 mg/day (0.15 mg/kg bw/day).
The most sensitive human health effects, that this ADI protects against, are seen consistently in the liver and thyroid in a number of studies using >98% pure CBD. This value also takes account of the lack of human-based long-term evidence and evidence regarding potentially vulnerable groups, which is applied here for this CBD isolate.[1]
Based upon the dossier of evidence provided by the applicant, the safety of the novel food was reviewed and evidence to reach a conclusion on safety provided. The evidence presented is consistent with evidence presented to support the development of a provision ADI of 10 mg/day for CBD of 98% purity or above. As such the provisional ADI should be applied to this novel food.
This is subject to the existing advice to consumers that pregnant and breastfeeding women and people taking any prescription medication should avoid the consumption of CBD. Consumers on regular medications should seek advice from a medical professional before using any type of CBD food product. In addition, children and prospective parents trying for a baby are advised against consumption of CBD, as are those who are immunosuppressed, due to remaining data gaps and residual uncertainties concerning the safety of CBD for these groups of consumers. These contraindications would also apply to this novel food.
The maximum safe exposure for healthy adults of 70 kg as identified in the provisional ADI is 10 mg per day. If the inclusion level of this CBD isolate leads to an intake per individual serving of each product type of 10 mg/day, only one product type per day should be consumed to ensure the ADI is not exceeded. Multiple intakes of products containing CBD on the same day should be avoided to support minimising exposure to below the provisional ADI.
3. Conclusions
The FSA and FSS have undertaken the assessment of isolated CBD and both concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake levels and the proposed use was not considered to be nutritionally disadvantageous.
These conclusions were supported by the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
-
in vitro bacterial reverse mutation test (Eurofins Munich),
-
in vitro mammalian cell micronucleus test (Eurofins Munich) and
-
90-day repeat dose feeding study (BSL Munich).
Abbreviations
Acknowledgements
The members of the ACNFP during the course of the assessment who were;Dr Camilla Alexander White, Dr Anton Alldrick, Dr Kimon Andreas Karatzas, Alison Austin, Professor George Bassel, Dr Mark Berry, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Catharina Edwards, Professor Susan Fairweather-Tait, Professor Paul Fraser, Dr Hamid Ghoddusi, Professor Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Mrs Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.
To note, interests were received from members of the ACNFP, Dr Alldrick declared a potential conflict of interest relating to his previous employment and was not present for discussions of CBD by the Committee. Emeritus Prof Harry McArdle declared his work with EFSA’s novel food Committee in considering data requirements for CBD. While not seen as a conflict, to avoid Prof McArdle being subject to information that would influence his EFSA work, it was agreed that he would not be present in discussions for CBD by the ACNFP but could supply comments for consideration by the Committee upon review of the minutes.
Updated on 29th January 2025 to include paragraphs in section 2.3 omitted in error. This is not a review of the assessment