This is a joint FSA and FSS publication
1. Introduction
Galacto oligosaccharide (GOS) is an authorised novel food in the UK (assimilated implementing regulation 2017/2470). In accordance with assimilated Regulation (EU) 2015/2283, the application for a change in the conditions of use of GOS (trade name Oligomate ®55N) as a novel food to increase the use level in food supplements, has been submitted in each nation of Great Britain (GB).
Whilst it was a Member State of the EU, the UK accepted the risk assessments of the European Food Safety Authority (EFSA) in respect of authorisations for regulated food and feed products. Since the end of the transition period, the FSA and FSS have adopted equivalent technical guidance and quality assurance processes to be able to undertake GB risk assessments for regulated product applications.
To ensure our regulatory systems are risk proportionate and resources are used effectively, the FSA and FSS have used the evidence submitted by the applicant and other information in the public domain, including the EFSA risk assessment opinion, to inform this safety assessment.
The FSA and FSS reviewers have evaluated the published EFSA risk assessment on the novel food and confirmed that this is appropriate for GB risk analysis. Consideration has been given to the processes undertaken to ensure the EFSA opinion is robust and whether there are any aspects that would require further review, such as specific issues for the countries of GB. The result of the assessment is that there is sufficient evidence of safety to conclude without requiring further risk assessment at this time.
This safety assessment represents the opinion of the FSA and FSS.
2. Details of other regulators opinions
The applicant, Yakult Pharmaceutical Industry Co., Ltd (Japan) is seeking authorisation of a change to the conditions of use of the novel food galacto-oligosaccharides (Oligomate ®55N) to increase the permitted use level in food supplements.
GOS are not a new novel food ingredient and under Regulation (EC) 258/97, GOS has been the subject of several novel food applications in the EU and applicable to GB. The first GOS product, Vivinal®, as marketed by FrieslandCampina, was exempt under the regulation due to a history of use prior to 15 May 1997. Three novel food GOS products have been subject to a substantial equivalence evaluation to Vivinal® by the competent authority of the EU Member State (The Food Safety Authority of Ireland) before their subsequent EU (and UK) authorisations in 2016 and 2017 (FSAI, 2016, 2017a, 2017b).
A fourth, Oligomate ®55N by Yakult Pharmaceutical Industry Co., Ltd was authorised in the EU (and UK) in 2013 following a substantial equivalence opinion by the Food Safety Authority of Ireland (FSAI) (FSAI, 2013).
GOS are authorised for use in several food and drinks categories for the general population and is added to improve prebiotic functionality of these products. This includes infant and baby food and formulas and food supplements.
In January 2021, EFSA published a risk assessment opinion on the safety of a change in the conditions of use of GOS for its use as a novel food ingredient in food supplements at the same higher use level as proposed in GB. This opinion has been reviewed by FSA and FSS risk assessors.
2.1. Methodology applied in the EFSA opinion
EFSA conducted the assessment of the novel food in accordance with the procedure as outlined in the EFSA scientific opinion ‘Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283’ (EFSA NDA Panel, 2016) and Commission Implementing Regulation (EU) 2017/2469.
2.1.1. Identity of the novel food
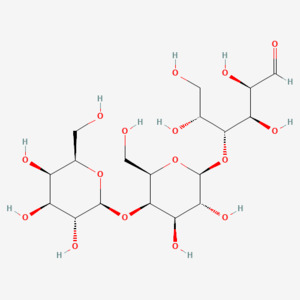
Oligomate ®55N is the trade name given to the novel food product in syrup form containing the GOS novel food ingredient. Oligomate ®55N comprises of ≥55% of the GOS novel ingredient by dry matter (DM) including lesser amounts of the saccharides lactose (≤40%), galactose (≥ 0.8%) and glucose (≤ 27%). The novel food is produced by enzymatic conversion, transgalactosylation and hydrolysis, of milk lactose by two β-galactosidase enzymes each produced from different yeast strains: Sporobolomyces singularis and Kluyveromyces lactis. GOS are carbohydrate molecules that vary in chain length and anomeric configurations comprising of galactosyl units linked by β-glycosidic bonds to a glucosyl unit. Synonyms of GOS include galacto-oligosaccharides, transgalactosylated oligosaccharides, transgalacto-oligosaccharides and oligogalactosyl-lactoses. GOS are a source of non-digestible dietary fibre (EFSA, 2010).
EFSA report that the major oligosaccharide GOS in the novel food, by configuration, is 4’-galactosyllactose (O-β-D-galactopyranosyl-(1→4)- O-β-D-galactopyranosyl-(1→4)- D-glucose) (Mwenya et al., 2005). The structure of the novel food is not changed by the current application made to the FSA and FSS and as such has not been subject to further assessment. The characterisation of the novel food ingredient below is provided per the existing authorisation and as assessed by EFSA.
GOS are characterised by the following information:
IUPAC name: (2R,3R,4R,5R)-4-[(2S,3R,4R,5R,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2,3,5,6-tetrahydroxyhexanal
CAS numbers: 66455-21-8 and 6587-31-1
Molecular formula: C18H32O16
Molecular mass: 504.4 g/mol
2.1.2. Production process
As detailed in the substantial equivalence opinion (FSAI, 2013), the production process starts with refined milk lactose that is enzymatically converted over two stages by way of two separately sourced β-Galactosidase enzymes chosen favourably for their ability to optimize enzymatic activity. A β-Galactosidase enzyme derived from the yeast Sporobolomyces singularis is used for trans-glycosylation of the lactose whereby producing GOS predominantly in the form of tri-, di and tetra-saccharides.
The second part of the process involves a second β-Galactosidase enzyme derived from the yeast Kluyveromyces lactis used to hydrolyse a portion of the lactose component into glucose and galactose. A final purification step takes place resulting in a syrup consisting of ≥55% GOS by dry weight with lesser amounts of the remaining components lactose, glucose and galactose.
The applicant did not propose any changes to the production process since the prior GOS substantial equivalence authorisation for Oligomate ®55N and assessment by the FSAI in 2013 (FSAI, 2013). EFSA concluded that the data provided on the production process was sufficient, and as such did not give rise to any safety concerns or hazards for management (EFSA NDA Panel, 2021). Upon review, the FSA and FSS agree with this decision and the changes to the conditions of use sought do not change that view.
2.1.3. Compositional information and specification
The novel food is produced by enzymatic conversion of lactose resulting in a purified syrup of 75% - 76% dry matter (≥55% GOS by dry weight or ≥41.25% GOS by wet weight) with remaining saccharide impurities consisting of lactose, galactose and glucose. The full specifications for GOS (Oligomate ®55N) per the current Union List authorisation and as assessed are shown in Table 1.
The FSA and FSS were satisfied that the composition of Oligomate ®55N complies with the selected parameters set out in the specifications in the Union List of Authorised Novel Foods under Commission Implementing Regulation (EU) 2017/2470 (EFSA, 2021).
The Union List specifications incorporate the amendment under Commission Implementing Regulation (EU) 2018/1023 to include the authorised microbial sources for the β-Galactosidase enzymes: Sporobolomyces singularis and Kluyveromyces lactis. The applicant provided a set of specifications specifically for Oligomate ®55N to the FSA and FSS and it was noted that these comply with the specification parameters in the Union List for GOS specifically as assessed by EFSA. Although not stated in the EFSA 2021 opinion, it is noted that the applicant demonstrated a higher minimum GOS (≥55% by dry weight) for Oligomate ®55N specifically than the GOS authorisation in the Union List (minimum 46% by dry weight). The current EU and UK authorisation that applies to GB, sets a minimum GOS content and as such permits at the higher GOS content for Oligomate ®55N.
Further compositional analysis was not reviewed by EFSA as the composition of the novel food remains the same as assessed and authorised previously. The FSA and FSS agree with this decision and the view that the changes in the conditions of use do not alter the previous assessment of the composition and specification or the conclusion that no safety concerns have been identified.
2.1.4. History of use
2.1.4.1. History of use of the source
GOS are a lesser known naturally occurring component of human breast milk (Eussen et al., 2021). GOS are relatively simple carbohydrate structures when compared to the more abundant Human Milk Oligosaccharides (HMO) and complex structures such as 2’-fucosyllactose (2’-FL) (Zivkovic & Barile, 2011). EFSA reports on the lack of or limited levels (1.9 - 3.9 mg/L) of GOS in human breast milk (EFSA NDA Panel, 2021, Sumiyoshi et al., 2004, Yamashita & Kobala, 1974). However, trace levels of GOS oligosaccharides during early lactation is reported across a number of more recent studies (Chen, 2015; Meeusen et al., 2024) and with an upper level of 5mg/mL (Newburg et al., 2016). It is noted that there is some disagreement in the literature for whether GOS is classified as a HMO due to various differences such as their separation from the HMO synthesis pathway (Meeusen et al., 2024). Besides lactose and lipids in human breast milk, total oligosaccharides account for around a third of its solid composition.
Bovine milk can contain trace levels of GOS, whilst bovine colostrum can contain up to 8.5mg/L of GOS (EFSA NDA Panel, 2021). Fermented milk products such as yoghurts can contain 0.03-0.58 % GOS due to naturally occurring microbial β-galactosidases and lactose-free milks up to 0.43 % GOS when these enzymes are used in the product manufacture (Ruiz-Matute et al., 2012; Toba et al., 1982; Villaluenga-Martinez et al., 2008)
2.1.4.2. History of use of GOS
Food industry production of GOS began in the 1980s (EFSA NDA Panel, 2021). The first GOS product Vivinal®, as marketed by FrieslandCampina, was exempt under the Novel Food regulation due to a history of use prior to 15 May 1997. Under Regulation (EC) 258/97, GOS have been the subject of several novel food applications from other producers producing an equivalent product using their own production methods.
Various novel food GOS products were subject to a substantial equivalence evaluation to Vivinal® before their subsequent authorisations (FSAI, 2016, 2017a, 2017b). This included the existing Oligomate ® 55N authorisation which has been marketed in the EU and UK since the FSAI 2013 opinion (FSAI, 2013). These authorisations include GOS added to foods such as infant and baby formulas to improve their prebiotic functionality.
GOS and Oligomate ® 55N are therefore not a new novel food for the GB market.
Outside of the EU and GB, other countries such as Australia, Canada (GOS as a dietary fibre), the United States and Japan (since 1995) also permit GOS products and various uses in foods.
2.1.5. Proposed use and anticipated intake
The target population is the general population (>3 years) which differs to the EFSA 2021 opinion (the general population) but is the same as the Commission Implementing Regulation (EU) 2021/900 of 3 June 2021. Under Commission Implementing Regulation (EU) 2021/900 of 3 June 2021 for the subsequent EU authorisation, a restriction was made to exclude infants and young children from this increased use level in food supplements. The applicant confirmed that this was due to the EU Commission’s more recent decision that food supplements taken by infants and young children, but not specific to GOS, should only occur where a medical or nutritional reason is confirmed by a doctor and for this reason would not be authorised for these groups at the higher level. The applicant therefore excluded the higher intakes in food supplements for infants and young children in their application to GB. The current use level in food supplements is also therefore still permitted for the general population in the EU and UK under Commission Implementing Regulation (EU) 2021/900 of 3 June 2021.
GOS have been permitted for use in various foods, beverages and food supplements by the general population since the original Vivinal® GOS authorisation and establishment of the Union List. Additionally, these were unchanged by the existing Oligomate ® 55N authorisation in 2013. The current maximum GOS use levels are defined in the Union List under Commission Implementing Regulation (EU)2017/2470. This application seeks to increase the maximum use level in food supplements for the general population (>3 years) per the 2021 EU authorisation. The uses assessed by EFSA (EFSA NDA Panel, 2021) are the same as those proposed to GB with the exception of being without the unrestricted target population, as detailed and explained above.
Table 2 below shows the existing maximum use levels for GOS as defined under Commission Implementing Regulation (EU)2017/2470. Table 3 shows the uses and maximum use levels to include the proposed increase in maximum use level for food supplements as defined under Commission Implementing Regulation (EU) 2021/900 of 3 June 2021.
Under the current EU and UK authorisation for GOS, as defined in the assimilated regulation (EU) 2017/2470 union list, GOS food supplements can be consumed by the general population (all age groups) up to a maximum intake level of 0.333 kg GOS per kg in the final food supplement. This corresponds to 4 g GOS per serving with a maximum of 3 servings per day and a maximum recommended daily intake of 12 g (EFSA NDA Panel, 2021).
The current assessment is focused with respect to the proposal for an increase to the maximum use level in food supplements for GOS. This results in an increase to the maximum use level from 0.333 kg GOS/kg (33.3%) to 0.450 kg GOS/kg (45.0%) in the final food supplement has been proposed for the general population, excluding infants and young children (≤3 years). This new maximum corresponds to a higher intake of 5.4 g GOS per serving with a maximum of 3 servings per day and a maximum recommended daily intake of 16.2 g. It was noted that in the EFSA opinion (EFSA NDA Panel, 2021), no safety concerns were raised for this proposed increase applicable to the general population.
The FSA and FSS noted that since GOS is not a new novel food, no formal exposure assessment was required by EFSA. EFSA concluded that the small increase in GOS consumption to the general population is not expected to significantly increase overall exposure and does not raise safety concerns (EFSA NDA Panel, 2021). Upon review, the FSA and FSS agree with this decision. Whilst a level of uncertainty regarding anticipated intakes and overall exposure from combined intakes (naturally occurring trace sources plus authorised uses) applies to GOS generally, the history of use of GOS in addition to the available information presented to the FSA and FSS, meant that no safety concerns were raised.
2.1.6. Absorption, Distribution, Metabolism and Excretion (ADME)
GOS are resistant to digestion by endogenous enzymes and largely reach the colon intact (90% in faeces) (Ambrogi et al., 2021). GOS are also categorised as bifido-genic as they are fermented and hydrolysed by particular colonic microbes, metabolising GOS into a carbon energy source for the bacteria to utilise.
ADME has not changed as a result of the proposed change to the conditions of use and therefore was not evaluated by EFSA in their opinion (EFSA NDA Panel, 2021). The FSA and FSS agree with this view and this aspect was not reviewed further.
2.1.7. Nutritional information
GOS are an established dietary fibre that are not digested or absorbed in the intestinal tract (EFSA, 2010). However, oligosaccharides are a prebiotic that are susceptible to anaerobic fermentation by intestinal microbes. GOS in particular, are a prebiotic known to modulate intestinal microbiome composition and have beneficial effects on metabolic and immune markers (EFSA NDA Panel, 2021; Lee et al., 2023). The small additional increase in dietary fibre by way of increased intake levels from food supplements to the specified population groups, does not raise concerns and is not expected to be nutritionally disadvantageous for consumers.
2.1.8. Toxicological information
Toxicological information was not applicable to the safety assessment for GOS due to a safe history of use for Vivinal® GOS and the subsequent substantial equivalence authorisation for Oligomate ®55N. Whilst not explicitly stated as such in the EFSA 2021 opinion, the opinion excluded any review of the toxicological information for GOS (EFSA NDA Panel, 2021) on the basis the change in conditions of use was unlikely to impact the toxicological behaviour of the novel food. Upon review, the FSA and FSS agree with this decision as proportionate to the GB assessment.
2.1.9. Allergenicity
Batch data on the protein content of Oligomate ®55N by dry matter, as assessed by EFSA, was reported to contain <0.1g/100 g (<0.1%) by Kjeldahl analysis.
EFSA (EFSA NDA Panel, 2021) report on the potential source of protein as the β-galactosidases enzymes which are otherwise removed during the purification steps of the production process. The EFSA evaluation concluded the likelihood of allergenic reactions to the ingredient GOS is expected to be low under the conditions of use. The FSA and FSS agree that the risk of allergic reactions remains low under the proposed conditions of use.
3. Other regulators opinions and conclusions
EFSA’s conclusions in their 2021 opinion were based on the original EU request submitted by the applicant before the exclusion for infants and young children were applied to increased food supplement intakes in the Commission Implementing Regulation (EU) 2021/900 of 3 June 2021.
EFSA concluded that the GOS novel food product Oligomate ®55N is an authorised GOS novel food that is substantially equivalent to the first GOS EU authorisation Vivinal® (FSAI, 2013). Oligomate ®55N consists of a purified syrup of 75% - 76% dry matter containing ≥55% GOS (≥41.25% GOS by wet weight), i.e., the novel ingredient, with remaining saccharide impurities consisting of lactose, galactose and glucose which are normal components of the diet (EFSA NDA Panel, 2021). The authorisation for GOS is set out in the Union List of Authorised Novel Foods under Commission Implementing Regulation (EU) 2017/2470. EFSA noted that GOS are therefore already authorised for use in several food and drinks categories for the EU and UK general population including food supplements.
The focus of the EFSA assessment was with respect to the proposed increase in maximum intake levels of GOS in food supplements of up to 0.450 kg GOS per kg of food supplement product (45.0%) for the general population. The increased intake levels at the proposed higher maximum level corresponds to a 5.4 g GOS per serving with a maximum of 3 servings per day and a recommended maximum daily intake of 16.2 g. The overall maximum increase from the prior maximum intake level of 0.333 kg GOS per kg (33.3%) amounts to an additional 1.4 g of GOS per serving. At a maximum of 3 servings per day, this amounts to an additional overall daily intake of 4.2 g for the general population at the higher intake levels. Since GOS are a non-digestible functional prebiotic dietary fibre, and the change in use level would result in a small increase in dietary fibre, EFSA concluded that this would contribute towards adequate fibre intakes and not put consumers at a nutritional disadvantage.
A 25g a day intake of fibre is considered to be an adequate intake for adults (EFSA, 2010). For children (over 1 year of age), limited intake data across European countries would suggest fibre intakes range between 6 and 46 g/day for children. Taking into account these limitations in the data on fibre intakes, upon review, the FSA and FSS agree with the conclusions made by EFSA that the higher intake of GOS from food supplements does not pose a nutritional disadvantage.
In review of the information provided by the applicant, the proposed increased use level of the GOS in supplements would result in an increased total daily intake from 48% to 65% of the adequate daily intake of dietary fibre in adults. This confirms consumption at the higher level is safe. The increase in maximum use levels for the general population (excluding infants and young children ≤3 years of age) did not give rise to any safety concerns by EFSA (EFSA NDA Panel, 2021). The FSA and FSS note that GOS are a naturally occurring trace component of foods and human breast milk. Notably, EFSA conclude on the safe history of use for GOS prior to 15 May 1997. GOS has been the subject of several prior authorisations and are concluded to be safe under the proposed changes. Upon review, the FSA and FSS agree with the conclusions made by EFSA.
4. Uncertainties and limitations
Regarding identity of the novel food GOS ingredient, the information provided on the exact configuration and chain length of the GOS saccharides present in the novel food as provided by the applicant is limited. EFSA report that the major oligosaccharide GOS by configuration is 4’-galactosyllactose which is also stated by the applicant in their dossier submission. However, in the absence of identity data provided by the applicant it is also likely that other GOS configurations β(1→6), β(1→4) or β(1→3) may also be present due to the enzymatic conversion by enzymes from specific microbes as reported more widely in the literature (Díez-Municio et al., 2014; Mei et al., 2022). The identity information did not raise safety concerns (EFSA NDA Panel, 2021). The FSA and FSS agree that collectively the identity information for the GOS novel food product (Oligomate ®55N) as reviewed by EFSA and previously by FSAI in 2013, was considered sufficient and did not give rise to safety concerns.
The FSA and FSS agree that the specific set of specifications provided by the applicant for Oligomate ®55N comply with the specification parameters in the Union List for GOS.
Regarding history of use, EFSA report on less recent evidence for GOS and its reported absence or trace level in human breast milk. The FSA and FSS note that more recent evidence in the literature has shown the presence of low levels of GOS in early lactation as a lesser known but naturally occurring component of human breast milk (Chen, 2015; Eussen et al., 2021; Meeusen et al., 2024; Newburg et al., 2016). It was noted by the FSA and FSS that there is some disagreement in the literature for whether GOS is classified as a Human Milk Oligosaccharide (HMO) due to differences such as the separation from the HMO synthesis pathway from the more well-known and highly abundant HMOs such as 2’-FL (Meeusen et al., 2024).
The limitations of the EU data for adequate fibre intakes in children are highlighted by EFSA. With respect to the applicant’s proposed exclusion of infants and young children following the EU implementing decision, the FSA and FSS are satisfied that fibre intakes from GOS at the proposed intake levels for the general population over 1 years of age does not raise safety concerns or pose a nutritional disadvantage.
There is uncertainty regarding the combined exposures to GOS with consideration for intakes from the newly proposed use level in addition to authorised uses and naturally occurring sources. Although this was not highlighted by EFSA, the FSA and FSS noted that since GOS is not a new novel food, no formal exposure assessment was required for the assessment. Whilst a level of uncertainty regarding anticipated intakes and overall exposure from combined intakes applies to GOS generally, the history of use of GOS in addition to the available information presented to the FSA and FSS was sufficient and exposure to the novel food did not raise concerns.
5. FSA-FSS conclusion for GB safety assessment
The application has been evaluated in line with the Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283 (EFSA NDA Panel, 2016), and assimilated Commission Implementing Regulation (EU) 2017/2469, for purposes of the GB safety assessment.
The conclusions of the EFSA opinion (EFSA NDA Panel, 2021), which have been reviewed in detail by the FSA and FSS for the purposes of the GB safety assessment, are considered appropriate and consistent within the uncertainties and limitations identified by EFSA.
6. Outcome of the assessment
The FSA and FSS have reviewed the applicant’s dossier, supporting documentation, and most notably the EFSA opinion (EFSA NDA Panel, 2021), and consider that there is sufficient evidence to conclude the safety assessment of GOS (Oligomate ®55N) without obtaining further information or conducting a further risk assessment.
The FSA and FSS conclude that GOS is safe under the proposed change to the conditions of use. The anticipated intake levels and the proposed increase in use levels in food supplements for the general population excluding infants and young children was not considered to be nutritionally disadvantageous. In addition, the FSA and FSS conducted a review of the available toxicological information on GOS and confirm it is non genotoxic. GOS are a naturally occurring trace component of foods and human breast milk and has been the subject of several prior authorisations including a degree of history of use prior to 15 May 1997. GOS are therefore considered safe under the proposed changes.
In making this assessment, the FSA and FSS were able to rely on sufficient scientific evidence to make a conclusion on safety with no further questions to the applicant, and therefore no further risk assessment activities are necessary.
Sufficient evidence was available in the literature to give the FSA and FSS confidence about the safety of this novel food, for example, where other national food safety authorities had positively assessed the application using the same risk assessment guidance and core legal requirements which apply in GB.
Applicants provided sufficient relevant information as requested by the FSA and FSS.
The FSA and FSS review did not find any issues of divergence from the EFSA guidance (EFSA NDA Panel, 2016) or mutual approaches or new scientific issues for consideration.
There were no other specific issues that would require an assessment for the UK or the nations of the UK.