This is a joint FSA and FSS publication
1. Introduction
In April 2022, Monterey Mushrooms, LLC (“the applicant”) submitted a full novel food application for the authorisation of UV-treated A. bisporus Mushroom Powder with increased vitamin D2. The novel food is produced by the exposure of Agaricus bisporus mushrooms to ultraviolet light which catalyses the conversion of endogenous ergosterol within the mushrooms to vitamin D2. The novel food is intended to be used as an ingredient in various food products, beverages and food supplements.
The FSA and FSS have undertaken a safety assessment for UV-treated A. bisporus Mushroom Powder with increased vitamin D2 under the novel foods legislation, assimilated Regulation (EU) 2015/2283. To support the safety assessment, the ACNFP provided the advice outlined in this opinion to the FSA and FSS.
The evaluation by the ACNFP assessed the food safety risks of the novel food and its production, in line with Article 7 of assimilated Commission Implementing Regulation (EU) 2017/2469. The regulatory framework and the technical guidance put in place by the European Food Safety Agency (EFSA) for full novel food applications is retained as the basis and structure for the assessment (EFSA NDA Panel, 2016).
Following the review by the ACNFP in September 2023, further information was requested from the applicant concerning the identity, the production process, the compositional information, the stability, the nutritional information, and allergenicity information on the novel food, in order to address information gaps in the initial dossier. The final advice from the Committee was agreed at the 167th meeting, allowing the FSA and FSS to complete the risk assessment.
The document outlines the conclusions of the FSA and FSS on the safety of UV-treated A. bisporus Mushroom Powder with increased vitamin D2 as a novel food.
2. Assessment
2.1. Identity of the novel food
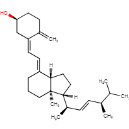
The novel food is a mushroom powder containing vitamin D2 which is produced by subjecting fungi of the species Agaricus bisporus, commonly known as cultivated mushrooms, to ultraviolet irradiation. Confirmation that A. bisporus mushrooms are used in the production process was demonstrated by high-performance thin-layer chromatography (HPTLC). Figure 1 shows the structural formula for vitamin D2.
Vitamin D2 is characterised by the following information:
IUPAC name
(3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-olCAS number 50-14-6
Molecular formula C28H44O
2.2. Production Process
The production process for UV-treated A. bisporus Mushroom Powder with increased vitamin D2 involves one raw material; A. bisporus mushrooms, which are grown and harvested in the USA under controlled environmental conditions in compliance with Regulation (EC) No. 852/2004 and within the principles of HACCP.
The mushrooms are diced (to increase available surface area) and exposed to UV light under appropriate conditions to achieve targeted levels of vitamin D2. The diced mushrooms are then dehydrated, ground and packaged in a temperature-controlled environment to control moisture levels in the product.
Prior to packaging, analyses are performed to ensure microbial safety as well as determine that the amount of vitamin D2 is within the food specification level of 125-463 µg/g.
Variability of the vitamin D2 content resulting from the variation of the effectiveness of the ultraviolet irradiation process due to factors including, exposure time, lamp properties, as well as variation in the raw material was noted. The conditions are managed to ensure the vitamin D2 level in the final product falls within the target range. It was also noted that the product may be blended again after final testing if the vitamin D levels do not meet client-specific requirements.
To ensure that the final product is homogeneous and that samples of the novel food tested for safety parameters such as moisture and vitamin D2 content reflected the composition of the batch tested, details of the blending and sampling processes were provided. A blender validation study involving 3 batches of vitamin D2 mushroom powder was undertaken to demonstrate the efficiency of the cone blender for creating homogeneous blends with targeted levels of vitamin D2 according to the specification. Information on how contamination is minimised during these stages of production and controls in the packaging stage to manage moisture levels was also provided.
The production process has characterised the potential hazards and the corresponding control measures are appropriate.
2.3. Compositional information
Results from five independent batches of UV-treated A. bisporus Mushroom Powder with increased vitamin D2 demonstrated that the novel food is produced consistently (Table 1). Vitamin D2 levels were assayed using high-performance liquid chromatography (HPLC). The analytical method is similar to that described by Phillips et al. (2011, 2012).
Certification was provided to demonstrate that the contract laboratories were accredited to perform these analytical studies. Where in-house analysis was utilised, full methodology and supporting validation documentation was provided.
It was noted that one batch was analysed using a different method of analysis, MPN-most probable number technique, which estimates the concentration of a microbe in a sample. No microbial growth was seen below the higher limit of detection for this test of 3MPN/g hence, all samples were considered to be within specification. Laboratory analysis showed that microbial contamination was appropriately managed and that controls were working effectively.
A Certificate of Analysis for testing of a wide range of pesticides showed that no residues were detected in the novel food.
Heavy metal levels were analysed and compared to established EU limits for the closest relevant finished product categories (Table 2). All analyses were below EU limits.
Three batches of the novel food were analysed for the presence of biologically inactive photoisomers (lumisterol and tachysterol) which are formed during the conversion of ergosterol to vitamin D2. These photoisomers are also formed endogenously in humans during cutaneous production of vitamin D following exposure to UV-B radiation from sunlight. The results demonstrated that only negligible amounts of these photoisomers are detected in the final ingredient (Table 3), and the amounts are lower than those in other UV-treated foods such as baker’s yeast and bread, which gave rise to no safety concerns in previous EFSA Scientific Opinions (EFSA NDA Panel, 2014, 2015).
The presence of aflatoxins was measured in six independent batches of UV- treated A. bisporus Mushroom Powder with increased vitamin D2. The maximum levels permitted in foods were taken from the closest relevant category (cereals and all products derived from cereals, including processed cereal products) detailed in assimilated Commission Regulation (EC) No 1881/2006, as amended, for aflatoxin B1 and the total sum of B1, B2, G1 and G2. Results demonstrated that the levels to be low and within established limits (Table 4).
The data presented indicate the novel food and any hazards present were appropriately characterised.
2.4. Stability
Stability tests were carried out on four batches for three years and on one batch for four years to test the stability of the vitamin D2 content under representative storage conditions (17°C-25°C and 40%-63% humidity). The results showed the vitamin D2 levels remained within the specified range and supported the proposed 3-year shelf life. Microbial analysis was carried out on the samples in the 3-year study at the start and end of the storage period, without any concerns identified. The results of both tests suggests that that the product is stable for up to 3 years under the recommended storage conditions.
The stability of vitamin D2 content was also tested within some foods that the novel food would be an added ingredient (fruit juice drink and cereal bar) under typical commercial storage conditions for the products. In addition to the analysis of vitamin D2 content, sensory parameters were tested. No significant degradation of vitamin D2 was observed throughout the test period of 14 days (the standard shelf life) for the juice and 3 months for the cereal bar. Sensory results did not indicate notable taste changes for the products.
On this basis, the data provided supports the stability of the novel food within the period of 36 months and storage conditions proposed.
2.5. Specification
The specification parameters for the novel food (Table 5) were assessed using internationally recognised methods or are otherwise determined using internally developed and validated methods.
The information provided is sufficient for the specification of the novel food, and appropriately characterises the novel food seeking authorisation.
2.6. History of Use
Wild edible fungi, including macro-fungi such as mushrooms, have been collected and consumed for thousands of years (FAO, 2004). Cultivated and wild grown A. bisporus mushrooms (the source of the novel food) have been consumed within and outside of the UK for a long time (FSAI, FSAI (Food Safety Authority of Ireland), 2017).
The novel food, UV-treated A. bisporus Mushroom Powder with increased vitamin D2 has no history of use in the UK. As the UV irradiation process is considered a novel process and the parameters for its operation will be slightly different between operators, each is subject to a separate review and authorisation.
UV-treated A. bisporus mushrooms have been consumed in the UK with their approval as a novel food ingredient since 2016 (FSAI, 2017). A similar ingredient produced by a different manufacturer using a different production process, vitamin D2 mushroom powder produced by homogenisation of mushrooms before exposure to UV light was authorised as a novel food in 2020 (EUR-Lex, 2020). Another form of vitamin D2 mushroom powder was considered safe under its intended uses (EFSA, 2021) and is authorised for use in the EU. The novel food that is subject to this application, uses a different process whereby mushrooms are first exposed to UV light before being dried and ground into a powder.
The history of use does not indicate any further areas for evaluation.
2.7. Proposed Use and Intake
The target population is the general population. For food supplements, the target population is for those aged 7 months and above.
The vitamin D2 mushroom powder is to be used in certain foods as well as food supplements as defined in GB food supplements legislation. A summary of the foods proposed to use the novel food as an ingredient and the maximum use levels for the novel ingredient in the form of a powder is provided in Table 6. These include breakfast cereals, dairy analogues, fruits and vegetable juices and non-alcoholic beverages.
The proposed maximum use levels of the novel food provide the following maximum additional intake of vitamin D2: for food products 2.25 μg/100g (15% of the adequate intake for vitamin D identified by EFSA), 1.125 μg/100ml (7.5% of the adequate intake identified by EFSA), for beverages, 15 μg/day for food supplements for age +1yr. 10μg/day for food supplements for infants 7-11 months, which is intended to provide the full adequate intake for vitamin D for the relevant age groups. This is based on EFSAs recent defined adequate intake (15μg/day for adults including children above the age of 1 and 10 μg/day for ages 7-11 months) as a replacement for nutrient reference value (EFSA, 2016).
Estimated intakes of vitamin D2 calculated based on the proposed use levels as presented in Table 6 were provided. The estimated total intake of novel food (mg/person/day) from all proposed food uses were calculated using the lowest dose of vitamin D in the powder i.e., 125μg vitamin D2/g powder, giving a maximum dose of 18μg powder per 100g.
Also provided was a summary of the estimated daily per kilogram body weight intake of the novel food from all proposed food categories in the UK by population group (Table 7). This was calculated with consumption data from the UK National Diet and Nutrition Survey (NDNS).
The maximum intended use level in Foods for Special Medical Purposes (FSMPs) is 15 μg vitamin D2/day, excluding those intended for infants. This is with the assumption that the powder would be the only source of vitamin D in the diet as many Foods for Special Medical Purposes replaces significant components in the diet. This is also the same maximum amount intended for total diet replacement for weight control and meal replacements for weight control.
Combined intake from the novel food and other sources was considered. Results from the summary of the estimated daily intake of vitamin D from the total diet (Table 8) is used to calculate the cumulative exposure of vitamin D from the total diet. It is concluded that based on these results, 29 μg/day for infants up to and including 11 months of age, and 25 and 26.3 μg/day for toddlers and other children, respectively, do not exceed the tolerable upper intake level (UL) of 35μg/day established by EFSA for infants aged 6 to 12 months of age and that of 50 μg/day for children aged 1 to 9 years. Similarly, the cumulative intakes of vitamin D from the total diet of 19.9, 30.5 and 23.3 μg/day among adolescents, adults, and elderly subjects, respectively, are well below the UL of 100 μg/day for each of these population groups.
Data on the likely impact of vitamin D exposure and the vitamin D2 vitamin, in particular from consumption of the novel food was noted. The additional consumption is predicted not to lead to increases above safe levels.
2.8. Absorption, Distribution, Metabolism and Excretion (ADME)
Two studies using UV-Irradiated mushroom products supplied by Monterey were undertaken. The first was a human study performed in a randomised, double-blinded, placebo-controlled 6-week study involving 38 healthy adults (14 males and 24 females) allocated 1 of 4 groups. The groups consisted of; a placebo control group receiving non-exposed mushroom powder, a group receiving UV-B-exposed mushroom powder providing 8.8µg/day, a group receiving 17.1 μg/day of vitamin D2, plus the placebo control dose and 1 supplement control group receiving vitamin D2 supplements providing 28.2 μg/day vitamin D2. All groups consumed similar meals containing 1 serving (87.9 g) of cooked white button mushrooms. The study measured serum 25(OH)D2, 25(OH)D3, 24,25- hydroxycholecalciferol [24,25(OH)2D3] and total 25(OH)D from blood samples taken at baseline, after 3 weeks and after 6 weeks.
The authors of the study concluded that that vitamin D2 from UV-exposed mushrooms is absorbed and metabolized to 25(OH)D2. They measured a small but significant decrease in serum 25(OH)D3. The authors hypothesise that this is a result of the study being undertaken in a sunny climate and so exposure to vitamin D from sunlight was a confounder in interpreting their results due to wider regulation of vitamin D levels by the body (Stephensen et al., 2012).
The second human ADME study involved a 12-week randomised study carried out during winter to minimise influence of environmental UV-B exposure, where 25 healthy adults consumed either UV-exposed mushroom extract (supplied by Monterey), vitamin D2 supplement or vitamin D3 supplement (all providing 50μg vitamin D2/day) in capsules once daily for 12 weeks. The study measured serum 25(OH)D, 25(OH)D2 and 25(OH)D3 from blood samples taken weekly throughout the study, to determine the bioavailability of the supplements. The study concluded that the mushroom extract was as effective at increasing and maintaining total serum 25(OH)D levels as supplemental vitamin D2 (Keegan et al., 2013).
The ADME information does not indicate any further areas of concern.
2.9. Nutritional Information
The novel food is mainly composed of approximately 50% carbohydrates, 33% protein, 20% dietary fibre, 9% ash, 3% fat, and less than 5% moisture. It also contains minerals and vitamins.
The nutritional content of mushrooms exposed to UV light is unchanged, with the exception of the intended increase in vitamin D2 content (Simon et al., 2011). Vitamin D2 from UV-irradiated mushrooms has been shown to be bioavailable in several human studies using 25(OH)D as an indicator (Keegan et al., 2013; Mehrotra et al., 2014; Outila et al., 1999; Shanely et al., 2014; Stepien et al., 2013), and that increases in 25(OH)D have also been observed in animal studies (Bennett et al., 2013; Calvo et al., 2013; Jasinghe et al., 2005; Koyyalamudi et al., 2009).
The ADME studies outlined bioavailability, and it was noted that while serum levels increased in some studies this was not necessarily related to changes in vitamin D status. This might be because of a difference in starting vitamin D status or exposure to sunlight. This was explored further in a study undertaken in the winter months.
Consumption of the novel food at the proposed use levels is not expected to be nutritionally disadvantageous for consumers.
2.10. Toxicological Information
2.10.1. Genotoxicity and Sub-chronic toxicity
Genotoxicity and sub-chronic toxicology studies were not provided on the basis that the impact of the novel process is on the vitamin D2 content of mushrooms. There are not expected to be specific concerns as a result of the conversion of the endogenous precursor to vitamin D2.
There are a number of authorised foods that are UV-treated; UV-treated baker’s yeast, UV-treated bread and UV-treated milk. There are also studies with ingredients similar to the novel food i.e. vitamin D2 mushroom powder produced by homogenisation of mushrooms before exposure to UV light (EFSA NDA Panel, 2020). No specific toxicological issues beyond vitamin D exposure were highlighted.
2.10.2. Human studies
A review of six human studies (Keegan et al., 2013; Mehrotra et al., 2014; Shanely et al., 2014; Stephensen et al., 2012; Stepien et al., 2013; Urbain et al., 2011) was provided with the conclusion that daily consumption of UV-B-exposed A. bisporus mushrooms providing up to 65 μg (2,600 IU) vitamin D2/day for 16 weeks is safe. There were not any associated reported adverse effects. The studies indicated Vitamin D2 from UV-B-exposed A. bisporus mushroom products was bioavailable, as evidenced by increased serum 25(OH)D2 (the direct metabolite of vitamin D2) concentrations.
2.11. Allergenicity
UV-treated A. bisporus Mushroom Powder with increased vitamin D2 is expected to have the same allergenic risk as that associated with consumption of A. bisporus mushrooms, as the ultraviolet treatment did not alter the composition of the mushrooms apart from the content of vitamin D2.
2.12. Discussion
The novel food is UV-treated A. bisporus Mushroom Powder with increased vitamin D2, produced by the exposure of Agaricus bisporus mushrooms to ultraviolet light which catalyses the conversion of endogenous ergosterol within the mushrooms to vitamin D2. The mushrooms are then dehydrated and ground into a powder.
The target population is the general population. UV-treated A. bisporus Mushroom Powder with increased vitamin D2 in food supplements is not intended to be consumed by those under the age of 7 months.
The novel food contains vitamin D2 content ranging between 125-463 μg/g and is intended to be used as ingredient to achieve a maximum dose of vitamin D2 of 2.25 μg/100 g for food products, and 1.125 μg/100 mL for beverages. In food supplements the proposed maximum dose of vitamin D2 is 15 μg/day in food supplements for the general population older than 1 year of age and at 10 μg/day in food supplements for infants from 7 to 11 months.
The proposed maximum vitamin D2 levels meet the Acceptable Intake level set by EFSA for these population groups. These use levels ensure that the required amount to be deemed as a source of vitamin D [as stipulated in Regulation (EC) No 1924/2006] would be met.
Combined intake of vitamin D2 from the novel food with that of the other dietary sources was considered to not exceed the upper limits for vitamin D previously established by the NDA Panel for infants (EFSA NDA Panel, 2018), and for children, adolescents and adults (EFSA NDA Panel, 2012).
3. Conclusions
The FSA and FSS have undertaken the assessment of the novel food, UV-treated mushrooms from Monterey Mushrooms Inc, and concluded that the novel food is safe under the proposed conditions of use and does not pose a safety risk to human health. The anticipated intake level and the proposed use in food and food supplements was not considered to be nutritionally disadvantageous.
These conclusions based on the information in the novel food dossier submitted by the applicant plus the supplementary information and could not have been reached without the following data claimed as proprietary by the applicant:
- annexes to the dossier which relate to the identity of the novel food, the production process, composition, stability, and the intakes assessment report.
Acknowledgements
The members of the ACNFP during the course of the assessment were;
Dr Camilla Alexander White, Dr Anton Alldrick, Ms Alison Austin, Dr Mark Berry, Professor George Bassel, Dr Christine Bosch, Professor Dimitris Charalampopoulos, Dr Meera Cush, Dr Cathrina Edwards, Professor Susan Fairweather-Tait, Dr Sophie Foley, Professor Paul Fraser, Dr Hamid Ghoddusi, Dr Andy Greenfield, Professor Wendy Harwood, Professor Huw D. Jones, Dr Kimon-Andreas Karatzas, Dr Ray Kemp, Dr Elizabeth Lund, Professor Harry J. McArdle, Dr Lynn McIntyre, Rebecca McKenzie, Professor Clare Mills, Dr Antonio Peña-Fernández, Dr Isabel Skypala, Dr Lesley Stanley, Professor Hans Verhagen, Dr Maureen Wakefield, and Professor Bruce Whitelaw.