- 1. Executive Summary
- 2. Background
- 2.1. Introduction and Scope
- 2.2. Commodity Description
- 2.2.1. Shell (table) eggs
- 2.2.2. The hen reproductive system
- 2.2.3. Anatomy and physical properties of the egg
- 2.2.4. Legal requirements and good practice in egg production
- 2.2.5. Egg processing
- 2.3. Consumption
- 2.4. Trade
- 3. Hazard identification
- 3.1. Methodology used for identifying hazards that can be found in EEPs
- 3.2. Literature review strategy
- 3.3. Creating the long hazard list
- 3.4. Shortlisting hazards for characterisation
- 3.5. Hazard identification results
- 3.5.1. Long hazard list
- 3.5.2. Agricultural contaminants
- 3.5.3. Allergens
- 3.5.4. Biocides
- 3.5.5. Environmental contaminants
- 3.5.6. Food additives
- 3.5.7. Feed additives
- 3.5.8. Metals
- 3.5.9. Microbiological hazards
- 3.5.10 Microplastics
- 3.5.11. Radionuclides
- 3.5.12. Pesticides
- 3.5.13. Veterinary medicines
- 3.5.14. Hazards shortlisted for characterisation
- 4. Hazard characterisation
- 4.1. Agricultural contaminants
- 4.2. Biocides
- 4.3. Environmental Contaminants
- 4.4. Metals
- 4.5. Microbiological hazards
- 4.6. Pesticides
- 4.7. Veterinary medicines and feed additives
- 5. Hazard Prevention, Mitigation and Controls
- 6. Conclusions
- 7. Uncertainties and Knowledge gaps
- Appendices
- Appendix I. UK consumption data
- Appendix II. Trade data
- Appendix III. Literature search terms used
- Appendix IV. Levels of radionuclides in eggs and egg products
- Appendix V. Levels of agricultural contaminants in EEPs
- Appendix VI. Levels of environmental contaminants in EEPs
- Appendix VII. Levels of metals in EEPs
- Appendix VIII. Levels of pesticide residues in EEPs
- Appendix IX. Levels of VMP and feed additive residues in EEPs
1. Executive Summary
This risk profile identifies and characterises the main hazards associated with shell eggs and egg products (EEPs) from domestic poultry species imported into the United Kingdom (UK). Liquid, dry, cooked and preserved egg products were in scope, while composite foods containing eggs or egg products were not.
The information is intended for the Department for Environment, Food and Rural Affairs (DEFRA) and its SPS Trade Assurance (UK Office), as well as the Food Standards Agency (FSA) and its Imports Market Access Assurance (IMAA) team. The document has three primary objectives:
-
Provide background on food safety concerns related to imported EEPs.
-
Support the assessment of market access requests to export EEPs to the UK.
-
Assist audit and assurance activities for EEP imports.
A comprehensive literature review and analysis of incident and alert data identified over 100 potential hazards that can be present in eggs. Through the use of inclusion criteria and expert judgement, 22 key hazards were shortlisted for characterisation.
The microbiological hazards that were taken forward to characterisation were Campylobacter spp., Listeria monocytogenes, and non-typhoidal Salmonella (Salmonella Enteritidis and Salmonella Typhimurium). Salmonella Enteritidis was the most frequently reported hazard in EEPs across all hazard types, and the main hazard involved in outbreaks associated with EEPs.
The chemical hazards that were characterised include: agricultural contaminants (aflatoxins, ochratoxin A (OTA) and pyrrolizidine alkaloids (PAs)), biocides (fipronil and chlorate), environmental contaminants (persistent organic pollutants (POPs) such as dioxins, polychlorinated biphenyls (PCBs), polychlorinated naphthalenes (PCNs), and per- and polyfluoroalkyl substances (PFAS)), melamine, metals (arsenic, cadmium, chromium, copper, lead, mercury, selenium) and a number of pesticides, veterinary medicinal products (VMP) and feed additives.
Risk factors for microbiological hazards include hygiene and biosecurity failures and the age and the size of the flock. Aflatoxin and OTA formation in feed is linked to a hot and humid climate, whereas PA concentrations are expected to be higher where nutrient availability is low and there is high soil moisture. For environmental contaminants and metals, proximity to anthropogenic sources of these contaminants are likely to impact the levels detected in eggs. Biocide, pesticide, feed additive and VMP residues are linked to the misuse of approved substances or illegal use of substances that are banned or not authorised for use in layer farms or egg processing environments.
Hazards may enter the egg during its formation or after it is laid. Pathogenic microorganisms such as Salmonella Enteritidis and chemicals such as mycotoxins, PAs, environmental contaminants, metals, pesticides and veterinary medicines follow the former route. These hazards are introduced to the egg within the reproductive system of the birds, either after environmental exposure of the animal, primarily via feed and water, or because they are part of the birds’ natural flora.
To mitigate microbiological hazards that are introduced to the egg during its formation, eradication and vaccination programmes, including for breeder flocks, are likely to be the most effective. Mitigation measures for chemicals in eggs and egg products are primarily related to the environment of the poultry farm, and the poultry feed and water. Monitoring programmes can also be effective.
Alternatively, hazards may enter the egg after it is laid, when it comes into contact with contaminated surfaces. Such hazards include microorganisms such as Salmonella Typhimurium, Listeria monocytogenes and Campylobacter spp. and biocides which are used in animal husbandry or egg processing environments.
Controls for microbiological hazards in eggs are predominantly concerned with the control of Salmonella but can be equally applied to mitigate other microbiological hazards. They include biosecurity measures, pest control, sampling and testing regimes, vaccination programmes, cleaning and disinfection programmes, product traceability, full documentation of activities undertaken, and records that can be audited independently.
In contrast to chemical hazards, microbiological hazards can be mitigated in industrial settings via heat treatment such as pasteurisation. Most egg products are pasteurised to destroy bacteria, however certain egg products may undergo milder processes due to heat sensitivity and shell eggs are not always pasteurised.
Regulatory measures are employed to control both chemical and microbiological hazards in eggs, and extensive regulations are in place in Great Britain (GB) and Northern Ireland (NI).
2. Background
2.1. Introduction and Scope
This risk profile identifies and characterises the main hazards associated with imported eggs and egg products (EEPs) of domestic poultry species that may be a concern for public health. Key controls, mitigation measures and relevant UK and EU regulations (applicable to Northern Ireland (NI)) are summarised along with common industry production methods and management processes, general UK consumption patterns and information on global production and trade. This information will be used by the Department for Environment, Food and Rural Affairs (DEFRA) UK Office for SPS Trade Assurance (UK Office) and the Food Standards Agency (FSA) Imports Market Access Assurance (IMAA) Team to:
-
Provide background information on potential food safety concerns relating to imported EEPs
-
Contribute to the overall evidence package used for assessment of specific third country market access requests to export EEPs to the UK
-
Support related audit and assurance activities
This risk profile does not assess risk and is not a risk assessment since exposure assessment and risk characterisation are not performed. This risk profile is not an exhaustive assessment of all potential hazards in EEPs, instead it describes the main human health hazards that may need to be considered in relation to the control of imported EEPs. This risk profile does not make public health recommendations or otherwise constitute public health advice. It is intended to inform on the hazards potentially associated with EEPs and to guide market access audit and assurance activities relating to imported EEPs. Identification of hazards in this profile does not necessarily indicate a present concern for public health from EEPs. However, further investigation such as risk assessment or review of controls or other specific audit activities may be required on the identified hazards before approving market access for EEPs. This risk profile does not address issues concerning fraud or authenticity unless there is an identified food safety consequence.
Two categories of eggs are included in this risk profile, namely shell eggs and egg products. Definitions are provided in Table 1.
Assimilated Reg. (EC) 853/2004, which lays down specific hygiene rules for food of animal origin, provides a different definition for eggs which excludes broken and cooked products (European Parliament, 2004). Since those were within the scope of the profile this definition was not considered appropriate.
While preserved eggs and egg products which may contain other ingredients are in scope, composite food containing eggs or egg products are not.
2.2. Commodity Description
This risk profile concerns EEPs, that are imported into the UK. However in this section legal requirements in the UK, good industry practice in the UK and UK egg assurance schemes (e.g. Lion Code and Laid in Britain) are discussed. The details outlined in this section may not be implemented as such in other parts of the world, but equivalent schemes aimed at ensuring the quality and safety of EEPs may be in place, and so the UK information is provided to aid comparison.
2.2.1. Shell (table) eggs
The following section focuses primarily on hen eggs as these are by far the most commonly imported EEPs (see section 2.4). Eggs from other species are discussed when information was readily available.
According to the Advisory Committee on the Microbiological Safety of Food (ACMSF), production of table eggs from other species, such as ducks, quail, geese, turkeys, ostriches and seagulls, varies between countries and typically such eggs are sold as small-scale alternative, niche or luxury commodities (ACMSF, 2016).
2.2.2. The hen reproductive system
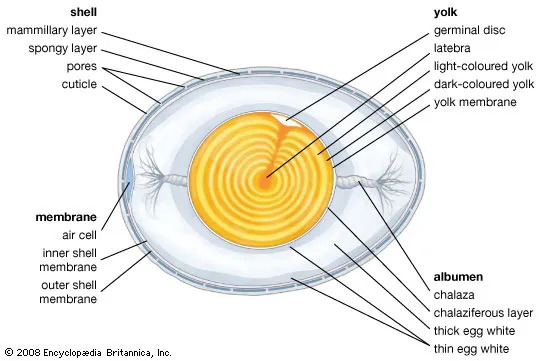
The hen’s reproductive system is made of the ovaries and the oviduct (Figure 1) (Kaspers, 2024). Eggs are produced from the inside out, starting with yolk formation in the ovaries and each successive layer being formed in different parts of the oviduct. This process takes 24-26 hours to complete (Kaspers, 2024). A representation of the egg layers can be seen in Figure 2.
Egg formation starts with the hens’ genetic material, called the oocyte or the ova. These are stored in follicles in the ovary (Figure 1A and B). The yolk forms around the blastodisc, which is a clear disc containing the female genetic information. The liver produces the egg yolk components (predominantly lipoproteins: triglycerides, phospholipids and cholesterol) which are transported to the ovary via the bloodstream (Nys & Guyot, 2011).
The yolk is then released from the ovary into the oviduct (Nys & Guyot, 2011). The opening of the oviduct, the infundibulum (Figure 1C), is where fertilisation of the egg takes place and where the vitelline membrane is formed (Nys & Guyot, 2011). This is a thin membrane that limits exchanges of the material between the yolk and the white and is a barrier against bacterial penetration. It is formed through specialised cells in the oviduct, ovaries and the liver.
The egg then moves further along the oviduct to the magnum (Figure 1D), where the albumen (egg white) is deposited. The role of the albumen is to protect the egg yolk and provide nutrients for a developing embryo (Nys & Guyot, 2011).
The next stage is formation of the two membrane layers (Figure 2) (Kaspers, 2024) in the section of the oviduct called the isthmus (Figure 1E) (Nys & Guyot, 2011). The membrane layers prevent the albumen from spreading out towards the shell. The inner membrane, the peri-albumen, may be involved in limitation of bacterial penetration.
Finally, the eggshell is formed, and this process can take 20-24 hours, the longest phase in the egg development process (Kaspers, 2024). Shell development occurs in three stages (Nys & Guyot, 2011):
-
Nucleation phase: eggshell membrane fibres are synthesised, and the organic components are laid down on the outer shell membrane.
-
Shell mineralisation: migration of the egg into the uterus (Figure 1F) where progressive hydration of egg albumen dilates the egg, creating its ovoid shape and allowing close contact with the uterine wall allowing calcium deposition to occur, forming the hard outer shell.
-
Termination of the mineralisation: calcium deposition stops and pigments comprised of porphyrins are secreted in the uterus which adds colour to the shell.
The expulsion of the egg (oviposition) by the oviduct is controlled by hormones and hormone-like substances (prostaglandins), that facilitate the uterine muscle contraction, and relaxation of the sphincter between the uterus and vagina (Nys & Guyot, 2011). The process lasts a few minutes.
A number of factors can affect the egg laying process. The diet needs to contain a large amount calcium along with other micronutrients to produce a healthy egg (Anonymous, 2018). Eggs with stronger eggshells are better able to resist pathogen penetration and internal content contamination. Hens under stress or on an inadequate diet may lay eggs that have thinner shells or reduced shell integrity, which can result in microcracks in the shell. This can result in contamination of the egg post-oviposition (Harage & Al-Aqaby, 2024).
2.2.3. Anatomy and physical properties of the egg
Eggs are an important food commodity that provide nutritional benefits, e.g. high-quality proteins (i.e. protein which contains all the amino acids required in the human diet), vitamins A, B12, D and E. They also offer functional benefits in food production e.g. the coagulant capacity of proteins, the foaming capacity of albumen proteins and the emulsifying capacity of the yolk (EFSA, 2014c). An egg is composed of the yolk (~30%), albumen or egg white (~60%) and shell membranes and shell (~10%) (Froning & Singh, 2024). Figure 2 shows the general structure of an egg.
The porous eggshell is predominantly composed of calcium carbonate and is formed in layers. The outermost layer, the cuticle, is formed last (USDA, 2000). The cuticle is a protein carbohydrate complex which protects the egg against dehydration and provides a barrier to many micro-organisms present on the surface of the egg by blocking the pores in the other layers of the shell (EFSA, 2005).
Underneath the shell are a pair of shell membranes; these are fibrous layers, which are composed of a protein core surrounded by carbohydrate. Many of the proteins in the fibres are antibacterial (EFSA, 2005).
Further in, the albumen is made of four viscous layers of varying thickness (Stadelman et al., 1995). Water is the major component of the albumen as well as numerous proteins, many of which can be antibacterial (EFSA, 2005).
The yolk is predominately composed of proteins and lipids, as well as vitamins and minerals, and it has a slightly acidic to neutral pH (USDA, 2000). Unlike the other component parts of the egg, it does not possess antimicrobial properties (USDA, 2021).
The approximate pH of the new-laid whole egg is 6.58, with the pH in the egg white being 7.96 and in the yolk 6.10 (FDA, 2023). The water activity(aW) is 0.97 (Schmidt & Fontana, 2007). Both the pH and the moisture in the egg change as a function of time and temperature (Y. B. Kim, Lee, et al., 2024). The pH in the egg becomes alkaline (~10) as the egg ages and cannot support microbial growth (USDA, 2021). These parameters are often favourable to bacterial growth as can be seen in Table 5,Table 7 and Table 8. However, pH and aW act synergistically with other parameters such as storage temperature, oxygen availability, salt levels, etc to determine whether microbial growth and/ or survival is likely.
The structure of the egg does not change between bird species. However, the proportions of the various egg components reflect the species, with the proportion of the shell varying the most (Nys & Guyot, 2011).
2.2.4. Legal requirements and good practice in egg production
In the UK and the EU (applicable in NI), egg production and distribution is governed by a number of regulations as discussed in this section. Additionally, the FSA appointed independent veterinarians to consider what criteria an industry food safety scheme would need to meet for the eggs to be considered ‘very low’ risk for Salmonella. They helped develop a science-based Matrix (i.e. a set of independent standards for production of shell eggs considered to be very low risk in relation to Salmonella) that can be used to facilitate consistent and independent assessment of all UK egg production assurance schemes and support the FSA egg consumption advice to vulnerable consumers (Unpublished).
The key elements of the matrix include:
-
Sampling and testing throughout the production chain, including at the hatcheries, feed mills, and laying flocks. The testing schemes should include environmental samples, raw materials and final product (feed or eggs) samples and should be conducted by suitably trained staff.
-
Vaccination programmes for both the breeding and laying flocks, use of antimicrobials exclusively to treat animal diseases and appropriate timing of use of products such as essential oils and acidification.
-
Pest control on the farm and in the feed mills.
-
Robust biosecurity controls, usually including physical barriers at the entrance to the farm, in addition to well-defined floor areas, dedicated footwear and overalls by house, and no access to pets.
-
Tamperproof traceability for class A eggs, including location and date of laying.
-
Cleaning and disinfection programmes, including between flocks
-
Fully documented temperature-controlled environment, from farm to retail, in line with the current recommendations on temperature control for eggs (constant temperature below 20 °C until shell eggs reach the consumers).
-
Records of activities such as sampling and testing, personnel training, any accidents such as feed spillage, pest control and temperature controls must be maintained at all times.
-
Independent auditing with clear documentation of “critical” standards must be carried out. Schemes must appoint certification bodies holding UKAS accreditation, or equivalent, having ISO17065 accreditation.
-
Clear guidelines on processes to be followed in the event of Salmonella being detected must be available, including processes of re-testing/contesting the result where appropriate, the process by which birds may be depleted and eggs sold/ disposed of, and vaccination programmes after a positive result has been received.
Any references to UK good industry practice refers to schemes adhering to the principles in the matrix. Not all steps in the safety scheme are prescribed in legislation. Such schemes may differ by country but for eggs to be imported into the UK, equivalent safety measures must be in place in exporting countries. Practices in countries other than the UK and the EU are also mentioned whenever possible.
2.2.4.1. Traceability and grading
In the UK and EU, egg traceability and record-keeping are mandatory. According to assimilated Reg. (EC) No 589/2008 and DEFRA guidance on egg marketing standards, eggs must be marked with the producer code (issued to producers upon registration) to identify and trace eggs, when they are sold as Class A or B, at public markets, or transported across EU Member State’s borders (DEFRA, 2020; European Commission, 2008a).[1]
In the UK, egg grading is governed by GB and Northern Ireland[2] (NI) legislation, and eggs are classified into two quality classes, A and B. Class A eggs are the highest quality eggs and are sold as shell eggs. Immediately after grading, they must be placed in packs and labelled with the best before date, along with other required information (DEFRA, 2020). Class B eggs are those that do not meet the quality thresholds of Class A eggs, or are Class A eggs that have been downgraded, and are typically used in the food industry for the production of egg products (BEIC, 2024a; European Commission, 2008a).
2.2.4.2. Cleaning and hygiene
In the UK, guidance on enforcement of hygiene regulations is provided by the FSA and Food Standards Scotland (FSS) (FSA, 2009a). The FSA matrix also requires robust cleaning and disinfection regimes to be in place. Requirements include maintaining poultry house, bird and egg storage cleanliness, controlling contamination arising from feed, ensuring water cleanliness, prevention of pest contamination, appropriate waste handling, appropriate control of pesticides and veterinary medicines and ensuring staff hygiene (FSA matrix, unpublished). Similar recommendations are made by the Codex (Codex, 1976). DEFRA provide a list of approved disinfectants that states which product to use and the concentration of the disinfectant that must be used (DEFRA, 2021).
2.2.4.3. Storage
Eggs in the UK have a shelf life (best before date) of 28 days. Before reaching consumers, eggs should not be chilled to ensure temperature variations do not affect the quality and safety of the product (DEFRA, 2020).
2.2.5. Egg processing
Shell hen eggs are marketed for direct retail sale, whereas egg products undergo further processing and are mainly intended for the industrial preparation of a variety of foods (such as pastries, dairy products, sauces, dressings, sweets and pasta) (EFSA, 2005). According to the European Food Safety Authority (EFSA), the eggs used for further industrial preparation are often those which do not meet the specifications for whole eggs (out-of-grade or downgraded products e.g. cracked eggs) (EFSA, 2014c). There are many types of egg products, the most common of which are liquid, dried, frozen and cooked whole eggs, egg yolk and egg whites. A summary of the key steps in these processes are shown in Figure 3, and a more detailed summary of the steps in production of liquid and dried egg is shown in Figure 4.
Many steps in the process are common across all egg production methods, these are detailed below.
2.2.5.1. Common steps in egg processing
Egg collection and transport
According to the British Egg Industry Council (BEIC), Lion Code egg producers and packers must adhere to specific regulations regarding collection and transport (see section 5.4.2). In particular, eggs should be transported to the packing centre within three working days of laying, necessitating at least two farm collections per week (BEIC, 2013). They must be stored at a consistent temperature below 20ºC to prevent surface condensation. If eggs are collected more than 24 hours after laying, a cooling device is required to keep eggs at an even temperature below 20oC (BEIC, 2013). Lion Code and Laid in Britain state that dirty, cracked, or broken eggs must be removed promptly from the collection system and cannot be sold for human consumption (BEIC, 2013; Laid in Britain, 2021).
Washing
Contemporary egg washing machines employ pressure sprays, rotating brushes, and a device that spins the egg to enhance the contact between the egg and the brush, thereby reducing potential damage to the eggs (USDA, 2015). The washing water temperature is set to 30 - 40oC (Shanghai Beyond Machinery, 2024).
According to Reg. (EC) No 589/2008, eggs should not be washed or cleaned as this can cause damage to the egg cuticle and shell, which acts as a barrier to bacteria (European Commission, 2008a). In the EU, only those Member States (MSs) that have granted permission for this practice can market washed table eggs (EFSA, 2014c). However, egg washing before processing is permitted in the EU and GB (Reg. (EC) No 853/2004, Reg. (EC) No 589/2008), provided that the eggs are immediately broken after washing (European Commission, 2008a; European Parliament, 2004).
EFSA suggests that washing reduces the microbial load on the egg surface, particularly foodborne zoonotic pathogens, but it does not prevent egg-based disease caused by micro-organisms which are internally present in eggs (EFSA, 2005). Additionally, washing can damage the cuticle of the eggs, supporting increased contamination within the egg, and increasing moisture loss (EFSA, 2005).
Candling
Candling is a technique that employs a bright light to detect and eliminate flawed eggs. This process involves mechanically spinning the eggs multiple times in front of a bright light to inspect their internal quality (Codex, 1976). The main objective of candling is to discard dirty, cracked, or unsuitable eggs prior to the crucial breaking step in shell egg processing (USDA, 2015).
Breaking and separating
Eggs used for processing should have fully developed shells with no breaks. Reg. (EC) No 853/2004 states that cracked eggs can only be used if they are delivered directly to the manufacturing site, where they must be broken as soon as possible to eliminate microbiological hazards, and that if processing is not carried out immediately after breaking, liquid egg must be stored either frozen or at less than 4°C. This storage period must not exceed 48 hours (European Parliament, 2004).
According to Reg. (EC) No 853/2004, eggs must be broken individually, and methods such as centrifugation or crushing, which were previously allowed, are now prohibited due to the high microbiological risk (European Parliament, 2004). The breaking process also includes separation of the yolk and albumen. EFSA states that the effectiveness of separation is influenced by factors including the storage temperature of the eggs, the freshness of the eggs, the type of machinery used and the rate at which the eggs are broken (EFSA, 2014c).
Reg. (EC) No 853/2003 states that eggs other than those of hens, turkeys or guinea fowl must be handled and processed separately, and that all equipment must be cleaned and disinfected before processing of hens’, turkeys’ and guinea fowls’ eggs is resumed (European Parliament, 2004).
Shell removal and filtration
The “Handbook of Egg Science and Technology” states that egg products are filtered to increase homogeneity and to remove eggshell debris (Mine, Guyonnet, et al., 2023). EFSA recommend that any shell debris remaining in the egg product should be immediately removed by sieving and filtration to prevent contamination and ensure complete homogenisation of the egg products (EFSA, 2014c). Reg. (EC) No 853/2004 states that the quantity of remaining eggshell, egg membrane, and any other particles in the egg product must not exceed 100 mg/kg of egg product (European Parliament, 2004).
Pasteurisation
According to the Codex, pasteurised liquid egg products should be cooled rapidly immediately after pasteurisation and refrigerated (Codex, 1976). EFSA states that exceptions are made for egg whites intended for drying (which are pasteurised post-drying) and for salted egg yolks (as refrigeration would increase yolk viscosity, hindering the pasteurisation process) (EFSA, 2014c).
Pasteurisation requirements for liquid whole eggs can vary by country. European heat treatments typically involve temperatures of 65 - 68 °C for 5 - 6 minutes for whole eggs and egg yolks. The United States of America (USA) requires a lower temperature of 60°C for at least 3.5 minutes (Lechevalier, Guérin-Dubiard, et al., 2017). EFSA state that egg whites undergo milder treatments (55 – 57 °C for 2 – 5 minutes) due to their higher heat sensitivity (EFSA, 2014c).
Storage and transport of egg products
Egg products are conditioned at consistent temperature prior to storage and delivery. EFSA recommend that the storage and delivery temperature of pasteurised liquid egg products should not exceed 4°C (EFSA, 2014c). The shelf life depends on the product type and packaging: two or three days at 4°C for bulk-packaged liquid egg products for the food industry, and up to sixty days for small packages for catering establishments, or consumers in some countries. EFSA also suggest that dried egg products and those with high sugar or salt content are generally recommended to have a shelf life of several months at ambient temperature (EFSA, 2014c).
2.2.5.2. Liquid egg production
Figure 4 shows the steps in typical production of liquid egg.
According to the British Egg Processors Association (BEPA), eggs with shells are usually; washed, rinsed, sterilised, candled, broken, separated automatically and checked for quality and flaws. The liquid egg product is then filtered, usually pasteurised, and packaged (BEPA, 2024).
If not used immediately in further processing, the liquid egg products must be refrigerated for storage and transport. BEPA state that whole eggs and yolks can be stored for two to six days at 4.4°C, egg whites can be stored for two to six days at 7.2°C, depending on the microbial quality of the product (BEPA, 2024).
According to the European Egg Processors Association (EEPA), there are two critical control points (CCPs) in liquid egg product processing. The first CCP is filtration and transfer (after breaking and/or before pasteurisation and/or before packaging), where there is a risk of physical hazards, in particular the presence of shell in the product or other foreign particles. This is addressed via visual examination of the filter. The second CCP is heat treatment and cooling, due to the potential of pathogenic microorganisms surviving. For this CCP, the control parameters are time and temperature of the heat treatment, in order to obtain at least 7 log10 reduction of Salmonella Enteritidis for egg yolk and whole egg product (EEPA, 2011).
2.2.5.3. Dried egg production
Figure 4 shows the steps in typical production of dried egg.
According to BEPA, eggs with shells are usually; washed, rinsed, sterilised, candled, broken, separated automatically and checked for quality and flaws. The liquid whole eggs and yolks are then clarified, filtered and pasteurised before drying. Glucose is normally removed from egg whites before drying to preserve the white colour and stabilise the product (BEPA, 2024).
The United States Department of Agriculture (USDA) states that two main methods are used for drying; spray drying (atomised liquid egg product is sprayed into a stream of hot air) and pan drying (egg whites are dried on pans to produce a flake-type or granular material). Spray drying is much more common, as it extends product shelf life, whereas pan drying is generally restricted to confectionary production (USDA, 2015).
Additional substances may be added to the dried egg to improve its properties, e.g. sugar or salt. This ensures the dried egg does not solidify, and sodium lauryl sulphate may be added to egg white to ensure aeration on reconstitution (BEPA, 2024).
BEPA recommend that the dried egg products should be cooled and stored at <10oC and kept sealed to prevent moisture ingress. Reconstituted egg should be stored at <10oC, and used within four days (BEPA, 2024).
During dried egg product processing, there are three CCPs according to EEPA. One CCP is the drying of egg powders where there is a potential for contamination or survival of pathogenic microorganisms. The parameter for control at this stage is the humidity of the powder with the aim to achieve aw<0.7. The next CCP is for the packing of egg powders, where there is a risk of inclusion of foreign bodies. The monitoring procedure involves sieving and metal detection. The remaining CCP is pasteurisation of powders where there is a microbiological risk of the survival of pathogenic organisms. The parameters to control for this stage are the time and temperature of the heat treatment (EEPA, 2011).
2.2.5.4. Frozen egg products
According to BEPA, eggs with shells are usually; washed, rinsed, sterilised, candled, broken, automatically separated and checked for quality and flaws. The liquid whole eggs and yolks are then clarified and filtered, before freezing at –23.3 - –40°C (BEPA, 2024).
Whole eggs are often mixed with sugar or salt to prevent gelation during freezing and thawing. Whipping agents such as triethyl citrate can be added to egg white to improve whipping, and citric acid can be added to yolk or whole egg products to limit greening of the yolk (BEPA, 2024).
BEPA suggest that frozen eggs can be stored at <–12.2°C for many years, but once defrosted should be stored at 4.4°C - 7.2°C and used within three days (BEPA, 2024).
As the production of frozen egg products is the same as production of liquid egg with a freezing step, the CCPs are as outlined in section 2.2.5.2.
2.2.5.5. Cooked egg products
Cooked egg products may be produced from shell eggs, or from other egg products such as dried or liquid egg, and includes omelettes, scrambled egg and hard-boiled eggs.
These egg products are produced via industrial cooking processes. BEPA state that omelettes and scrambled eggs are packed into sealed containers, whereas hard boiled eggs are peeled then packed in a preservative solution of sodium citrate and 0.1% sodium benzoate or potassium sorbate to inhibit mould growth (BEPA, 2024). The cooked egg products should be stored either frozen or refrigerated, dependant on the manufacturer’s instructions (BEPA, 2024).
2.2.5.6. Preserved eggs
Preserved eggs are a speciality in Asian countries, such as China, and modified traditional processing methods are often used to produce them. In China, fresh raw eggs are pickled in an alkaline solution that contains, salt, tea and metal ions such as copper, iron and zinc. The process takes place at room temperature and lasts for more than 40 days (Xue, Han, et al., 2022). Metals such as lead oxide used to be added to preserved eggs to assist with the gelation of the egg, however this practice has been banned in China due to health concerns associated with lead (Xue, Han, et al., 2022).
2.3. Consumption
2.3.1. Consumption estimates of EEPs in the UK
Chronic and acute consumption estimates for egg were obtained using data from the Diet and Nutrition Survey for Infants and Young Children (DNSIYC) and National Diet and Nutrition Survey (NDNS) for all age groups between 4 months and 95 years (Gov UK, 2013, 2019).
The DNSIYC includes infants and children between 4 and 18 months and was carried out in 2011. The NDNS includes participants from 18 months – 95 years, and the data used is from years 1 to 11 of the NDNS. The NDNS rolling programme is a continuous, cross-sectional survey designed to collect detailed, quantitative information on food consumption, nutrient intake, and nutritional status of the general population in UK private households. The survey covers a representative sample of around 1000 people per year.
Appendix I presents detailed chronic and acute consumption[3] data for foods containing ≥ 5% egg, including consumption of both whole eggs (e.g. boiled or fried eggs) and composite products containing EEPs (e.g. quiche, omelette and cake). While composite products are out of scope of this risk profile, this data is considered more representative of UK EEP consumption pattern. In addition, HMRC trade data (2019-2024) shows that that over 60% of EEPs imported into the UK are egg products rather than whole eggs, with egg products predominately used for onward processing into composite foods (HMRC, 2024a).
NDNS and DNSIYC food codes (and their definitions) used to estimate consumption are listed in Appendix I. Consumption estimates using these food codes show that infants (4-18 months) are the highest chronic consumers of eggs on a per kg bodyweight per day basis, consuming 1 g/kg/bw/d (mean) and 4.2 g/kg bw/d (97.5 percentile). Regarding acute consumption, again on a per kg bodyweight per day basis, the highest consumers are also infants (4-18 months) who consume 3.1 g/kg bw/d (mean) and 11 g/kg bw/d (97.5th percentile).
For consumption in g/person/day when comparing with the average weight of a medium or large egg (53g-63g and 63g-73g, respectively), high consumers (97.5th chronic) are estimated to eat 1-2 eggs per day depending on age group (BEIC, 2024b).
2.3.2. Consumer behaviour
The Food and You survey is a consumer survey commissioned by the FSA to provide evidence on consumers’ self-reported food-related activities and attitudes. The survey has been running on a biennial basis since 2010 and provides data for England, Wales and NI (FSA, 2019). In the survey 75% of respondents reported eating cooked eggs at least once per week, including 8% reporting that they eat them every day. 86% of respondents reported never eating raw or uncooked eggs. No information on egg products was included within the report.
The DEFRA Family Food Dataset for UK Household Purchases in 2021 – 2022, shows that an average of 2 eggs were purchased per person per week in UK households. This is much lower than the UK consumption estimates provided from the NDNS and DNSIYC (Appendix I). This may be due to the reporting of purchasing rather than direct consumption or related to the averages taken for the DEFRA Family Food Dataset.
According to the exposure assessment in the FSA ‘Salmonella risk profile of UK-produced hen shell eggs’, which used industry data, the consumption of eggs per person per year in 2021 was estimated to be 202 (FSA, 2023b).This has increased from 195 per person per year in 2016 and differs to the data reported from the DEFRA Family Food Dataset. This may be due to averages taken as part of the DEFRA Dataset per household.
2.4. Trade
2.4.1. UK Exports
Non-hatching eggs are traded under the trade codes 040721, 040729, 040790, 0408 and 350211 (as defined in section 2.1). UK EEPs export data was extracted from the UN Comtrade database (United Nations, 2024). The UK exports EEPs to over 75 countries, however 98.84% of total exported EEPs from the UK are imported by 15 countries.
A total of 137,821 tonnes (t) were exported from the UK 2016-2022. The majority (49,7000 t) were exported to the Netherlands, representing approximately 36% of the total export volume measured between 2016 and 2022. The second largest volume of exports was to Ireland (43,952 t, 32% of volume), followed by France (17,552 t; 13% of volume).[4] The 15 highest recipients of UK EEPs exports are summarised in Appendix II.
2.4.2. UK Imports
Import data from His Majesty’s Revenue and Customs (HMRC) shows that between 2016 and 2022 the UK imported a total of 458,934 t of EEPs, representing an average of approximately 65,562 t of EEPs per year (HMRC, 2024a). Of all imported EEPs approximately 70% are egg products rather than whole eggs.
The top three countries that the UK imported most EEPs from were Netherlands (55.9%), Ireland (11.8%) and Germany (6.6%). Data on the 15 countries the UK imported the largest volume of EEPs from is presented in Appendix II.
2.4.3. Global Trade
Global export data was extracted from the UN Comtrade global database using the commodity codes listed above (section 2.4.1) for the period 2016-2022 (United Nations, 2024).
The top five countries exporting eggs globally between 2016 and 2022 in the order of the highest trade volume were India (17,000,280 t), Netherlands (3,649,581 t), Türkiye (1,772,048 t), Poland (1,748,527 t) and Malaysia (1,299,403 t). Data is presented in Appendix II.
3. Hazard identification
3.1. Methodology used for identifying hazards that can be found in EEPs
A systematic literature review method was followed for the data collection in relation to the identification of hazards that can be found in poultry EEPs. The process followed the PRISMA guidelines on data collection (PRISMA, 2020). Data synthesis and statistics were not in scope for this work.
The primary question asked during the searches was “What hazards are found in poultry eggs or egg products that may pose a risk to human health?”.
Publications were included if they met any of the following criteria with respect to EEPs:
-
Contained information on prevalence of human health related hazards
-
Reported on results of surveillance or monitoring
-
Risk assessments or exposure assessments discussing hazards
Publications falling within any of the categories below were not included:
-
Method development studies that did not include a surveillance component
-
Experimental studies in food or feed that involved artificial inoculation of the samples
-
Articles discussing the effectiveness of policies on hazards elimination or control
-
Articles discussing authentication of organic products
-
Articles reporting on non-human health related hazards
-
Articles reporting on interventions to control hazards in animals
-
Articles that did not discuss incidence in any form
-
Articles that were not accessible
To note, some of these papers were still considered for later stages in the work, e.g., for hazard characterisation or exposure pathways.
For certain hazard groups such as allergens and biocides the criteria were further defined as follows:
-
Allergenicity of EEPs themselves was not in scope, although it is discussed briefly in later sections of this report. However, information on allergenic proteins from different species that can be found in EEPs were in scope
-
Biocides approved for use in the food industry for cleaning and disinfecting purposes. It is expected that misuse of the products is the main way in which residues can be found in the food commodities. For this reason, the scope of the searches was broadened to cover experimental studies on how such substances can be transferred into EEPs
3.2. Literature review strategy
3.2.1. Database searches
A comprehensive search was conducted across EBSCO, Pubmed, Scopus and Springer using an FSA internal tool that can search all 4 databases simultaneously (FSA, 2024c). The databases were searched for publications between January 2000 and June 2024.
The search terms were structured so that the articles returned would be limited to incidence of human health related hazards that can be found in poultry EEPs. An example of how such a search term was used can be found below. Not all the terms were necessarily used in all searches.
Example of search terms:
[poultry AND egg* AND hazard AND (surveillance OR survey OR alert OR notification OR outbreak) AND (food OR consumption OR human health) NOT (eggplant OR parasite)]
More details on the search terms can be found in Appendix III. Searches were conducted for general hazards and for each of the relevant hazard groups as identified and discussed in the following sections of this report.
3.2.2. Article selection process
All articles returned by the searches were scanned for relevance to the scope of the risk profile. An article was included when:
-
The title or abstract included the word “egg”
-
After reading the abstract, the inclusion criteria described above were satisfied.
3.3. Creating the long hazard list
The long hazard list consisted of any hazard that was identified either via the literature reviews or FSA and FSS incidents and outbreak data or alert information included in the FSA Risk Likelihood Dashboard (RLD), Food Akai or the FERA horizon scanning tool (FERA, 2024; FoodAkai, 2024; FSA, 2024e). EU and UK reports on monitoring levels of veterinary medicines and pesticides were also included.
3.3.1. Results of the literature review
Full articles included in step 3.2.2 were accessed and scanned for information on hazard incidence in EEPs. Once a hazard was identified as reported in eggs it was added to the long list of hazards, allocated a unique ID and grouped under one of the following hazard groups:
-
Agricultural contaminants (mycotoxins and plant toxins)
-
Allergens
-
Biocides
-
Environmental contaminants
-
Feed additives (added to the feed for purposes other than medicating the animals)
-
Food additives
-
Metals
-
Microbiological
-
Microplastics
-
Pesticides
-
Process contaminants
-
Radiological
-
Veterinary medicines
3.3.2. Food Safety Alerts
The FSA Risk Likelihood Dashboard which contains information on food alerts from the UK, Australia, Japan, FDA and EU (Rapid Alert System for Food and Feed (RASFF)) was scanned for alerts raised in relation to EPPs between January 2019 and June 2024. Any unique hazards identified were added to the long hazard list.
Food Akai and the FERA horizon scanning tool data were also scanned in the same way for the period January 2019 to June 2024 (FERA, 2024; FoodAkai, 2024).
3.3.3. FSA and FSS Incidents
FSA records for incidents and outbreaks involving EEPs since January 2013 and FSS records for incidents and outbreaks between January 2019 and June 2024 were scanned for unique hazards that were used to generate the long hazard list.
3.4. Shortlisting hazards for characterisation
The hazard longlist was refined prior to hazard characterisation to focus resources on hazards where there is evidence of concern in relation to EEPs. Hazards that are banned in the UK, were highlighted but not characterised as an assessment had led to the ban and no further information could be added at present. Hazards were also not taken forward for characterisation when it was concluded that not enough information was currently available to complete the task.
The shortlisting was conducted using the criteria in Table 2. In the event of any ambiguity during the shortlisting of a hazard, expert judgement was used to determine inclusion.
To note for food additives, if they were approved under Reg. (EC) 1338/2008 for use in EEPs specifically they were excluded from further characterisation as they are expected to be found in the products they are approved for (European Parliament, 2008).
3.5. Hazard identification results
3.5.1. Long hazard list
3.5.1.1. Literature searches
The literature searches produced 1399 hits (after duplications removed). Of those, 1216 articles contained the word “egg” in the abstract. Articles that did not contain the word “egg” but instead contained the word “foods” or “foodstuffs” were checked to ensure relevant articles were not discounted, and 9 extra articles were selected. At the abstract scan stage 209 were considered to be relevant, although the full article content was not accessible for 32 of those publications. The rest were taken forward to the full paper assessment stage as shown in Figure 5. These papers fed into the long hazard list alongside the alert, incident and outbreak data and reports from regulatory sources such as EFSA, FAO etc . A total of 135 unique hazards were included in the long list.
3.5.1.2. Outbreaks, Incidents and alerts
Food safety alerts and FSA and FSS incidents were also scanned to confirm relevance. Composite products were not in scope for this risk profile and therefore any alerts/ incidents referring to hazards in composite products were not included in the long hazard list. This principle was applied to the extent that the product was adequately described in the notification.
Table 3 contains data on the FSA and FSS incidents (notifications about non-compliance instances) reported between 2013 and 2024, noting any that also qualified as outbreaks (human cases reported). FSA records for incidents and outbreaks involving EEPs since January 2013 and FSS records for incidents and outbreaks since January 2019. Records from the onset of recording until June 2024 were scanned for this information, as per section 3.3.3. Alert data for 2019-2024 is also included; this information is obtained from the FSA Risk Likelihood Dashboard as per section 3.3.2, it includes food alerts from the UK, Australia, Japan, FDA and EU (RASFF).
3.5.2. Agricultural contaminants
Nine agricultural contaminants were identified as potential hazards in eggs during the literature review. The hazard detected most commonly was mycotoxins, specifically aflatoxins and ochratoxin A (OTA).
Aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) are mycotoxins produced by fungi of the genus Aspergillus which are found especially in areas with hot and humid climates (EFSA, 2020b). OTA is produced by various fungi of the genus Aspergillus and Penicillium (EFSA, 2020b).
Several studies investigated the levels of aflatoxins and OTA in food of animal origin including eggs (Adegbeye, Reddy, et al., 2020; Keutchatang, Tchuenchieu, et al., 2022; Omar, 2021; L. Wang, Zhang, et al., 2018). Other mycotoxins, including fumonisins, patulin T2/HT2 and zearalenone, were discussed in papers relating to mycotoxins in food, however no specific evidence of detection in eggs was reported. One alert for mycotoxins in eggs was identified, however the nature of the mycotoxins was not specified.
Maximum legal limits for aflatoxins in poultry feed are set in GB and NI under regulations GB 2015/255 and 2002/32/EC respectively (European Parliament, 2002; Gov UK, 2002, 2015c). Other toxins, including OTA are recommended to be kept below specific limits in poultry feed in EU (2006/576/EC) (European Commission, 2006).
In addition to mycotoxins, evidence was found in the literature review that pyrrolizidine alkaloids (PAs) can be present in eggs (P. P. J. Mulder, López, et al., 2018). PAs are toxins exclusively biosynthesised by plants to provide a defence against herbivores. (EFSA, 2011a; FERA, 2014).
The information from the alerts, literature review, and regulations on poultry feed was sufficient to indicate that certain toxins should be subject to hazard characterisation, specifically aflatoxins, OTA and PAs will be characterised.
3.5.3. Allergens
There are 14 regulated allergens which must be declared if present in food. One of these is eggs, and hence any food containing eggs or egg products could pose a risk to allergic individuals (FSA, 2014). However, the allergenicity of EEPs themselves is out of scope for this profile, as it relates to the inherent properties of the proteins in the egg (Caubet & Wang, 2012), rather than a hazard which may be introduced to the egg or egg product.
Allergens could be introduced in EEPs either intentionally or unintentionally due to cross-contamination in the supply chain. The only allergen identified in the literature review or alerts was sulphur dioxide (sulphites), with alerts raised due to insufficient labelling. Under Reg. (EC) 1169/2011 sulphur dioxide must be declared as an allergen if the levels are above 10 mg/kg or 10 mg/litre (calculated in terms of the total sulphur dioxide (SO2)) (European Parliament, 2011; Gov UK, 2011).
Sulphur dioxide will not be characterised in this context, as while allergens in general are a concern to consumers, there is no information that indicates insufficient labelling of allergens is a particular concern in EEPs when compared to other commodities.
Sulphur dioxide is authorised as a food additive in GB and NI (see section 3.5.6 for discussion).
3.5.4. Biocides
Biocides are substances which are intended to control harmful organisms via chemical or microbiological action (HSE, 2024b). Limited information was found in the literature review to indicate the presence of biocides in eggs.
In a review of the disinfectant usage in egg production in the UK, around 11 biocides or groups of biocides were identified, but levels of biocides in eggs were not reported (Wales et al., 2021).
Hydrogen peroxide was involved in an incident in the UK that affected egg products. Hydrogen peroxide is approved in assimilated Reg. (EC) 2015/1730 (European Commission, 2015; Gov UK, 2015a) in UK and NI for use in several biocidal product types (PT), including PT3 – veterinary hygiene disinfectants, and PT4 – food and feed disinfectants (HSE, 2024h, 2024d).
Hydrogen peroxide used in aseptic packaging evaporates before filling with food and no residues in food are expected (ECHA, 2015). Disinfected distribution systems for drinking water are also flushed before being refilled with drinking water. Subsequently, no MRLs are required for hydrogen peroxide as a biocidal product because it is not persistent, no systemic health effects are observed and because of its high reactivity (ECHA, 2015). As such, hydrogen peroxide has not been characterised.
Two pesticides identified in eggs, fipronil and chlorate, also have biocidal uses and residues detected in eggs are likely to have arisen from biocidal uses, so these chemicals have been characterised as biocides.
3.5.5. Environmental contaminants
The literature review identified several environmental contaminants associated with EEPs. The majority of these contaminants are persistent organic pollutants (POPs), which are defined as ‘organic substances that persist in the environment, accumulate in living organisms and pose a risk to our health and the environment’ (ECHA, 2024).
In the literature review, the most commonly identified hazards were dioxin and dioxin-like substances (polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and dioxin-like and non-dioxin-like polychlorinated biphenyls (PCBs) (BfR, 2010; Castellani, Manzoli, et al., 2021; EFSA, 2012c; Hoang, Traag, et al., 2014). They are predominantly present in the environment as a result of industrial processes (COT, 2010). Levels of dioxins and dioxin like PCBs are regulated in EEPs in GB and NI (GB 1881/2006) (EU 2023/915), and alerts have been raised in the UK and the EU for multiple instances of detection in eggs (European Commission, 2023a; Gov UK, 2006).
Per and polyfluorinated substances (PFAS) have been detected in eggs (DTU National Food Institute, 2023; Zafeiraki, Costopoulou, et al., 2016). PFAS are a class of over 12,000 fluorinated substances that have been produced since the 1940s and which are, or have been, used in a broad range of consumer products and industrial applications (COT, 2022a). Levels of four PFAS are regulated in eggs in the EU (NI) in Reg. (EC) 2023/915 (European Commission, 2023a).
Polychlorinated naphthalenes (PCNs) are a group of 75 chemicals of the class of chlorinated polycyclic aromatic hydrocarbons (EFSA, 2024f), some of which (PCN2-PCN8), are considered persistent organic pollutants (POPs) under the Stockholm Convention (EFSA, 2024f). FERA investigated contaminants in specialty (non-chicken) eggs for sale in the UK, and detected PCNs in all of the samples (FERA, 2017a). EFSA concluded that eggs and egg products are a major contributor to PCN in the EU diet (EFSA, 2024f).
Organophosphate esters (OPEs) (also known as organophosphates (OPs)) have been detected in eggs (D. Li, Zang, et al., 2020). OPs are a group of chemical compounds widely used in pesticides, veterinary and human medicines, biocides, and more in recently fire retardants (COT, 1999, 2019). A review paper looking at OPE levels in food globally found levels in animal products, including eggs, were generally significantly lower than non-animal products (J. Li, Zhao, et al., 2019), and a study in the UK showed concentrations of OPEs in eggs were the lowest of all food groups (Gbadamosi et al., 2022).
Melamine has also been detected in poultry eggs (Alizadeh, Hosseini, et al., 2023). Melamine is used mainly in the synthesis of resins for manufacturing (WHO, 2008). It is also a metabolite of the pesticide cyromazine (EFSA, 2010c). Maximum legal levels for melamine are set in all foods in GB and NI under assimilated Reg. (EC) 1881/2006 and Reg. (EC) 2023/915 (European Commission, 2023a; Gov UK, 2006).
Limited information was also found in the literature review about the presence of chlorinated paraffins (CPs) in eggs (Ding, Zhang, et al., 2021). EFSA undertook a risk assessment for CPs which found fish, and liver and fatty tissue of mammals to have significantly higher levels than eggs (EFSA, 2020c).
The information from the alerts, literature review, and regulations was sufficient to indicate that certain environmental contaminants should be subject to hazard characterisation, specifically dioxins and dioxin-like substances, PCBs, PFAS, PCNs, and melamine.
3.5.6. Food additives
Food additives are substances which are added to food to perform a specific technological function, exerting an effect on a food. They include colours, stabilisers, preservatives and sweeteners (FSA, 2024b). The definition of a food additive is given in retained Reg. (EC) No 1333/2008 as 'any substance not normally consumed as a food in itself and not normally used as a characteristic ingredient of food, whether or not it has nutritional value, the intentional addition of which to food for a technological purpose in the manufacture, processing, preparation, treatment, packaging, transport or storage of such food results, or may be reasonably expected to result, in it or it’s by-products becoming directly or indirectly a component of such foods" (European Parliament, 2008).
Limited information was found in the literature review to indicate food additive residues may be present in egg and egg products.
One paper was identified which showed that benzoic acid, potassium sorbate, sodium benzoate, sodium sorbate, sorbic acid and sulphur dioxide/sulphites were detected in samples of egg products (D. Li, Zang, et al., 2020). All of the food additives detected in the study are approved preservatives in processed foods in UK under assimilated Reg. (EC) 1333/2008 with the exception of sodium sorbate (European Parliament, 2008; Gov UK, 2008).
Alerts due to undeclared sulphur dioxide were identified for preserved duck eggs, quail eggs in vinegar, and in eggs (unspecified) due to insufficient labelling as sulphur dioxide is an allergen (see section 3.5.3).
The permissible additives have not been considered in the hazard characterisation. Sodium sorbate has not been considered in the hazard characterisation as there is very limited evidence of presence in eggs, and no evidence to suggest that egg is a primary vehicle for this hazard.
3.5.7. Feed additives
Feed additives are defined under Reg. (EC) 1831/2003 as substances which are deliberately added to feed to perform functions such as meeting nutritional requirements, improving feed quality, animal performance and productivity (European Parliament, 2003). Results were found in the literature review for feed additives with cyromazine, lasalocid, maduramicin, monensin, narasin, nicarbazin, salinomycin and semduramicin reported in egg and egg products.
Cyromazine is an insecticide (also a pesticide or a veterinary medicine for some animals other than poultry), it has not been considered in the hazard characterisation for feed additives as there is very limited evidence of presence in eggs, and no evidence to suggest that egg is a primary vehicle for this hazard. Cyromazine is not authorised for use in poultry GB or NI (European Commission, 2024b; FSA, 2023a).
The remaining feed additives are all antiprotozoal agents - coccidiostats and histomonostats, used for the treatment of poultry coccidiosis (an intestinal infection) and histomoniasis (an infection of the liver and cecum) (ACAF, 2007). Antiprotozoal agents are predominately regulated as feed additives in the UK, although some are also regulated as veterinary medicines (European Commission, 2009c, 2009b; Gov UK, 2009b, 2009a). For consistency they are considered together with veterinary medicines in the hazard identification and hazard characterisation.
3.5.8. Metals
Heavy metals such as lead, arsenic, cadmium and mercury are present naturally in the environment (EFSA, 2024d). Human activities such as farming, industry or pollution can increase the levels of metals in the environment. Contamination of the food chain by metals might occur from the environment or during food processing and storage (EFSA, 2024d).
The literature search indicated that a range of metals, including mercury (Hg), antimony (Sb), arsenic (As), cadmium (Cd), lead (Pb), copper (Cu), nickel (Ni), chromium (Cr), manganese (Mn) and selenium (Se) may be present in egg and egg products. Several studies show metals including Pb, Cd, As, Hg, Cu, Ni and Cr are present in eggs (Iqbal et al., 2023; Kabeer, Hameed, et al., 2021; Salar-Amoli & Ali-Esfahani, 2015; Zergui et al., 2023).
A number of metals (lead, cadmium, mercury, arsenic, tin) are controlled in a range of commodities with prescribed maximum levels (MLs), however there are no MLs for metals in eggs (GB 1881/2006) (EU 2023/915) (European Commission, 2023a; Gov UK, 2006).
The information available was sufficient to indicate that metals in eggs should be subject to hazard characterisation. Arsenic, cadmium, chromium, mercury and lead were identified for hazard characterisation based on evidence of their presence in eggs and their higher toxicological concern (Tchounwou, Yedjou, et al., 2014).
In addition, copper and selenium were identified for hazard characterisation as they can pose a human health risk at certain levels, and specific concerns related to their presence in eggs have been identified. Selenium is regulated in animal feed in Reg. (EU) 2022/1459 (European Commission, 2022a) and eggs are a significant contributor to human exposure (EFSA, 2024c). Copper is used as a plant protection product (PPP) which has been implicated in eggs in alerts (EFSA, 2023b).
3.5.9. Microbiological hazards
Several microbiological hazards were identified in the literature review. Salmonella was the most frequently reported in a large number of papers and identified in the highest number of incidents and alerts for both Salmonella Typhimurium (1/195) and Salmonella Enteritidis (31/195) over the last 5 years. The Salmonella serovar was not always identified in the alerts. In 2016, the ACMSF undertook a risk assessment of Salmonella, in eggs and concluded that it was the microorganism, primarily Enteritidis, of most significant concern with respect to egg content contamination (ACMSF, 2016). EFSA concluded in 2014 that Salmonella Enteritidis was the only pathogen currently posing a major risk of egg-borne diseases in the EU, with S. Tymphimurium rarely being associated with eggs in Europe (EFSA, 2014c). In the same report, EFSA concluded that S. Typhimurium may be of more relevance in ducks.
Listeria monocytogenes was also identified as a microorganism of concern in eggs in the literature review. EFSA report studies indicated the presence of L. monocytogenes in caged hen flocks and noted its ability to contaminate eggshells and its presence in liquid eggs at breaking plants (EFSA, 2014c). Other studies also show that Listeria monocytogenes are often present in egg breaking plants, for example one study showed that 8.5% of raw egg products were contaminated (Rivoal, Fablet, et al., 2013). Egg associated outbreaks of L. monocytogenes are not frequently reported, however, an outbreak in the USA in 2017-2019 resulted in multiple human cases and 1 death (CDC, 2020). The implicated product was hard boiled eggs intended for food processors and restaurants.
Campylobacter is also considered by EFSA to present a concern with regard to EEPs. They report studies indicating that Campylobacter can be present on egg shells as well as studies showing presence in raw egg products (EFSA, 2014c). Campylobacter are prevalent in many domestic animals including cattle, sheep and pigs but their main reservoir is live poultry, including chicken, ducks, geese, turkeys and ostriches (Sahin et al., 2002). Recent studies have reported a prevalence of Campylobacter on eggshells up to 94.6% depending on the housing of the birds (Casalino, Bozzo, et al., 2022; Gharbi, Bejaoui, et al., 2022).
For the other microbiological hazards identified, such as Bacillus spp, Escherichia coli and Cronobacter sakazakii, there was minimal information available in the literature review. As a result, the hazards were excluded from characterisation primarily based on eggs not being a primary food vehicle for human exposure. The only other microbiological hazards considered for characterisation was Clostridium botulinum and Avian Influenza. C. botulinum was implicated in an incident in the UK in which over 150 laying hens died, however there is no evidence of any transfer to egg and egg products for consumption. Regarding Avian Influenza, a recent FSA risk assessment indicated that poultry products represent negligible (chicken and turkey) to low (ducks and geese) risk and no further information has been available since the publication of this assessment (FSA, 2024d).
Therefore, Campylobacter spp, L. monocytogenes, and Salmonella spp are the microbiological hazards identified for characterisation in EEPs.
3.5.10 Microplastics
Plastic pollution has been widely recognised as a global environmental problem. However, the potential risks from exposure to smaller plastic particles i.e., micro- and nano-plastics in humans are yet to be fully understood (COT, 2021a). There is no international definition for microplastics (COT, 2021a; EFSA, 2016a). The Committee on Toxicity (COT) describes microplastics as “synthetic particles or heavily modified natural particles with a high polymer content that are submicron-mm in size (0.1 to 5,000 µm or micrometres)” (COT, 2021a). In Europe, European Chemicals Agency (ECHA) defines microplastics as “particles containing solid polymer, to which additives or other substances may have been added, and where ≥ 1% w/w of particles have (i) all dimensions 0.1µm ≤ x ≤ 5mm, or (ii) a length of 0.3µm ≤ x ≤ 15mm and length to diameter ratio of >3” (European Chemicals Industry Council, 2020).
Microplastics can be divided into two categories as: primary microplastics, intentionally produced in micro size for certain purposes (e.g., microbeads in cosmetics) and secondary microplastics formed in the environment due to fragmentation of larger pieces of plastic (e.g. plastic bags) (COT, 2021a). Secondary microplastics over the course of time can be fragmentated into smaller particles to form nanoplastics (EFSA, 2016a).
EFSA state that currently available data indicates presence of microplastics in seafood, beer, honey and table salt (EFSA, 2016a). No data was reported for eggs, even though eggs were targeted in their search terms (EFSA, 2016a).
One publication showed the average content of microplastics in hen eggs sampled to be 11.67 particles/egg, with most particles being spherical and 50-100μm in size. More microplastics were found in the egg yolk than the egg white, which the authors attribute to microplastics being lipophilic (Liu, Chen, et al., 2022).
No incidents related to microplastics in eggs were reported during the timeframe in scope, and microplastic levels are not regulated in eggs. Particulates in general are a potential risk to consumers although there is no information suggesting that eggs are of particular concern for particulates when compared with other commodities. With regards to micro and nano plastics, the human health effects are not well defined and therefore a conclusion on consumer risk related to eggs could not be reached. Therefore micro/nano plastics were not characterised, but their presence in eggs cannot be ruled out.
3.5.11. Radionuclides
Radionuclides, also known as radioactive materials or radioactive isotopes, are unstable forms of elements that emit radiation as they undergo radioactive decay (Gov UK, 2024a). Natural radionuclides detected in eggs in Europe include, K-40, U-238, Ra-226, Pb-210, Po-210 and Th-228 (Cinelli, De Cort, et al., 2019).
Studies on radionuclide contamination in eggs have focussed on Ra-226 and Cs-137 as these are the most commonly identified types (Fathabadi, Salehi, et al., 2017; Shah & Abdeljawad, 2024). Ra-226 is a natural radionuclide formed in the U-238 decay series, and hence is present in many foods, drinking water and dust (which can be inhaled). Cs-137 is an artificial radionuclide, present in the environment as a result of authorised or accidental release from the nuclear power industry or weapons testing (Cinelli, De Cort, et al., 2019).
A study on levels of radionuclides following the Chernobyl incident showed Ba-140 and Li-140 were the main radionuclides detected in the shell after the chickens were fed with contaminated grass (Cosma, 2002).
In the UK, the Radioactivity in Food and the Environment (RIFE) report is published each year which brings together monitoring results for radioactivity in food and the environment. The main aim of the RIFE programme is to monitor the environment and diet of people living or working near nuclear and selected non-nuclear sites, to estimate the amount of radioactivity the public is exposed to. In the most recent report for 2022 eggs were shown in most cases to have levels below detection limits (CEFAS, 2022).
In summary some radionuclides have been detected at trace levels in eggs including those such as Cs-137 that are derived from nuclear discharge and weapons testing, and others that are present naturally.
No incidents related to radionuclides in eggs were reported during the timeframe in scope. Radionuclides in food are controlled under assimilated Council Reg. (Euratom) 2016/52, with levels for EEPs covered by ‘Other food except minor food’ (Gov UK). Radionuclides in general are a potential risk to consumers although there is no information suggesting that eggs are of particular concern when compared with other commodities. Therefore, radionuclides will not be characterised.
3.5.12. Pesticides
Pesticides include insecticides, fungicides and herbicides, which are used to control pests, weeds and diseases (HSE, 2024g).
The use of pesticides is widespread and varied. From the literature review, 28 pesticides were identified in eggs, with four (chlorate, chlordecone, cyromazine, and fipronil) specifically implicated in alerts, incidents and monitoring. Pesticide residues in food are controlled according to maximum residue levels (MRLs) in GB and NI (assimilated Reg. (EU) 396/2005) and there is evidence that pesticide residues may exceed MRLs in eggs (European Parliament, 2005; Gov UK, 2005). According to the 2021 UK quarterly surveillance results on pesticide residues in food, one non-compliant egg sample with an MRL exceedance was reported, giving a non-compliance rate of 0.76% (1 out of 132 egg samples) in 2021 (DEFRA, 2022a).
Overall, pesticide residues are readily detectable in eggs, and they may be present at levels exceeding relevant MRLs. Therefore, hazard characterisation was performed for pesticides, generally with a focus on those pesticide residues that were found to exceed MRLs in eggs in reports by GB or EU regulatory authorities.
3.5.13. Veterinary medicines
Veterinary medicines products (VMP) are substances used for the treatment or prevention of disease, or altering physiological functions in animals (CEFAS, 2024). From the literature review, around 28 VMP residues (including those reported as feed additives) were detected in eggs.
In the feed additives section (3.5.7), those in scope are antiprotozoal agents - coccidiostats and histomonostats. These are predominately regulated as feed additives in GB and NI (under assimilated Reg. (EC) 124/2009), but some are also regulated as veterinary medicines in GB and NI (under assimilated Reg. (EC) 37/2010), and there is inconsistent reporting of incidents and alerts for these substances (European Commission, 2009c, 2009b; Gov UK, 2009b, 2009a). For consistency, both veterinary medicines and antiprotozoal feed additives are considered together in this section.
VMP residues in food and feed are controlled according to MRLs in GB (VMD, 2024b) and NI (European Commission, 2009c). The UK and EU undertake an annual monitoring programme for VMP residues in food. Non-compliance in eggs where levels of VMP residues exceeded the MRLs were reported.
The annual non-compliance rates for coccidiostats in the UK were between 0.0% - 0.61 % from 2019 to 2023, with an average of approximately 700 samples analysed per annum. For other VMPs, the annual non-compliance rates were between 0.0% - 0.072 % from 2019 to 2023, with an average of approximately 1,500 samples analysed per annum (VMD, 2024d).
According to the EU annual reports on VMP residues (2019-2021), the annual non-compliance rates for antibacterial substances in eggs were between 0.17-0.26%, with approximately 5,500 samples per annum. The non-compliance rates for anticoccidials in eggs from 2019-2021 were between 0.21 - 0.42% with approximately 5500 samples per annum (EFSA, 2024b).
Residues of a range of veterinary medicines can be detected in eggs. These may be at levels exceeding the MRL or they may be not authorised for use in laying hens or poultry. The information was sufficient to indicate that residues of veterinary medicines should be subject to hazard characterisation generally, and specifically, those that have been found to exceed the MRL by GB or EU authorities or are otherwise unauthorised in eggs.
3.5.14. Hazards shortlisted for characterisation
The 135 unique hazards on the long hazard list were taken through the shortlisting process described in Table 2. A total of 22 individual hazards or hazard groups as shown in Table 4 were selected for characterisation.
4. Hazard characterisation
4.1. Agricultural contaminants
The agricultural contaminants shortlisted for characterisation are mycotoxins (aflatoxins, and OTA) and plant toxins (PAs). Conclusions on the concerns regarding the potential agricultural contaminant presence in EEPs are presented in section 4.1.4.
4.1.1. Aflatoxins
4.1.1.1. Hazard route
Aflatoxins are mycotoxins produced by two species of Aspergillus, a fungus found especially in areas with hot and humid climates (EFSA, 2024a). Humans can be directly exposed to aflatoxins through foods such as nuts and rice as a result of fungal contamination. Animals can be exposed through feed, leading to the presence of aflatoxins in products of animal origin (EFSA, 2020b).
Animal feeds such as extracted copra, peanut cake, sunflower cake, corn gluten, rice bran, cotton seed, palm kernel and soybeans were identified by EFSA as significant carriers of aflatoxins, in particular AFB1 in feed materials. The likelihood of contamination of the feed materials relates to the geographic origin (i.e., humidity and high temperature) (EFSA, 2004a). Aflatoxins do not bioaccumulate in fatty tissue, as such accumulation in the egg has only been identified under experimental conditions with extremely high aflatoxin concentrations in the feed. Under less extreme conditions, a carry-over into the eggs is unlikely (BfR, 2013).
A review paper was published by MacLachlan in 2011 to estimate the transfer of contaminants in animal feedstuffs to certain food products. The transfer factor was calculated by dividing residue concentration measured in the animal commodity of interest (in this case, eggs) to the residue concentration measured in the animal diet, including any contribution from ingested soil. Transfer factors for AFB1 from animal feeding stuffs into chicken eggs and quail eggs were reported as 0.00042 and 0.0005 respectively (MacLachlan, 2011).
4.1.1.2. Hazard characterisation
The International Agency for Research on Cancer (IARC) classified aflatoxins as Group 1 Carcinogens: Carcinogenic to humans (IARC, 2024). Aflatoxins are rapidly absorbed; distribution and accumulation is in the liver where the major metabolism and toxicity takes place (EFSA, 2020b). Aflatoxins can pass through the placenta in humans, and in experimental animals, metabolites of aflatoxins were found in the liver of foetus and mother (EFSA, 2020b).
Different potency for carcinogenesis were identified for aflatoxins, however due to insufficient data to derive cancer potency factors, EFSA assumed the carcinogenic potency of AFB1/2 and AFG1/2 to be similar to AFB1 (EFSA, 2020b). Both the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and EFSA did not consider it appropriate to establish a health-based guidance value (HBGV) due to genotoxic carcinogenicity (EFSA, 2020b; JECFA, 2016). EFSA derived a benchmark dose lower confidence limit (10%) (BMDL10) value of 0.4 µg/kg based on hepatocellular carcinoma incidence in rats. They adopted a margin of exposure (MOE) approach, which indicates that an MOE of 10,000 or higher would be of low health concern (EFSA, 2020b).
A summary of the levels of aflatoxin in EEPs identified in the literature, are given in Appendix V, with AFB1 detected at levels up to 168 μg/kg in whole egg (L. Wang, Zhang, et al., 2018). A study on prevalence, level and health risk assessment of mycotoxins in fried poultry eggs showed that the maximum level of AFB1 in the egg yolk was 1.45 μg/kg and in the egg white was 1.23 μg/kg (Omar, 2021), suggesting a similar distribution across the yolk and white.
No maximum level (MLs) have been set for aflatoxins in eggs and egg products. The MLs in assimilated Reg. 1881/2006 and Reg. (EU) 165/2010 for total aflatoxins (sum of AFB1, AFB2, AFG1 and AFG2) for specified foodstuffs (e.g. groundnuts, cereals) range between 4 - 15 μg/kg (European Commission, 2010; Gov UK, 2006).
4.1.2. Ochratoxin A
4.1.2.1. Hazard route
Ochratoxins are mycotoxins produced by various fungi of the genus Aspergillus and Penicillium, e.g., A. ochraceus, and P. verrucosum which grow in hot and humid areas. Humans can be directly exposed to OTA through food such as grains and dried fruit as a result of fungal contamination, or animals can be exposed through feed, leading to the presence of OTA in products of animal origin (EFSA, 2020b, 2020c). OTA can be found in modified forms. Due to the altered structures of the modified OTA, detection by the analytical methods aimed at the parent toxins may not be successful. However, these modified forms may contribute to the overall exposure and toxicity (EFSA, 2020c).
OTA is frequently present in the feed of food-producing animals (EFSA, 2020c). In the 2004 EFSA review, it was reported that chickens absorb approximately 40% of OTA after exposure, however later data included in the 2023 EFSA review indicated higher bioavailability (99% absorption) in laying hens (EFSA, 2004b, 2023d). Metabolites in poultry are mainly eliminated through excreta (EFSA, 2023d). Poultry species appear to eliminate OTA faster than monogastric mammalian species, resulting in a low OTA accumulation in the blood and tissues (EFSA, 2023d).
Transfer of OTA from feed to eggs is negligible (up to 2 mg OTA/kg of feed) and only occurs when OTA intake is very high (OTA at 10 mg/kg bw) (EFSA, 2023d). It was estimated that 0.11% of OTA in feed was transferred to eggs (EFSA, 2023d).
4.1.2.2. Hazard characterisation
IARC classified OTA as Group 2B: Possibly carcinogenic to humans (IARC, 2024). Kidney toxicity in different animal species and kidney tumours in rodents were reported with OTA exposure, and were selected as critical endpoints (EFSA, 2020d; JECFA, 2007). OTA is genotoxic both in vitro and in vivo; however, the mechanisms of genotoxicity are unclear. Direct and indirect genotoxic (e.g., oxidative stress) and non-genotoxic modes of action might contribute to tumour formation.
Due to uncertainty regarding the mode of action for kidney carcinogenicity, it is inappropriate to establish a HBGV and an MOE approach was applied by EFSA. For non-neoplastic effects, a BMDL10 of 4.73 µg/kg bw/day was calculated from kidney lesions observed in pigs, and an MOE of 200 being of low health concern (EFSA, 2020c). For neoplastic effects, a BMDL10 of 14.5 µg/kg bw per day was calculated from kidney tumours seen in rats with an MOE of ≥ 10,000 being of low health concern (EFSA, 2020c).
A summary of the levels of OTA in EEPs identified in the literature are given in Appendix V, with OTA detected at levels up to 2 μg/kg in the whole egg (Adegbeye, Reddy, et al., 2020; Keutchatang, Tchuenchieu, et al., 2022).
No MLs have been set for OTA in eggs and egg products. The MLs in assimilated Reg. (EU) 1881/2006 and Reg. (EU) 2022/1370 for OTA for certain foodstuffs (e.g. spices, cereals) range between 0.1 – 80 μg/kg (European Commission, 2022c; Gov UK, 2006).
4.1.3. Pyrrolizidine alkaloids
4.1.3.1. Hazard route
PAs are toxins exclusively biosynthesised by plants; more than 660 different PAs have been discovered. It has been estimated that approximately 6,000 plant species worldwide, may contain PAs such as Asteraceae (e.g., gordolobo yerba) and Fabaceae (e.g., borage) (COT, 2008; FSA, 2020). PA concentrations are expected to be higher when nutrient availability is low and increasing soil moisture might lead to higher PA-concentrations in the roots (Codex, 2014).
In the review by EFSA, bee derived products (e.g., honey) were the only foodstuffs of animal origin assessed, due to a lack of comprehensive studies for other animal products (EFSA, 2011a). PAs or their metabolites may indirectly contaminate eggs following exposure of poultry to PA-contaminated feed or if PA sources are present in forage fields. However, exposure to PAs from eggs is at a significantly lower level compared to plant-originated foodstuffs (e.g., herbs and spices) (EFSA, 2007b).
Based on the limited information, carry-over of PAs from animal feed into eggs was reported to be approximately 1% (EFSA, 2011a). Different PAs were shown to be transferred in different ratios into the eggs; this was investigated in a study by Eroksuz et al (2008), from which [EFSA] calculated transfer ratios of PAs into eggs between 0.22-1.08%, depending on the PAs (EFSA, 2011a; Eroksuz, Ceribasi, et al., 2008).
Different transfer ratios of PAs were reported in another feeding study in laying hens, using herbs with different PA profiles (P. Mulder, de Witte, et al., 2016). Overall transfer rates for the sum of PAs were estimated between 0.02% and 0.23%, depending on the type of PAs in the feed. The study also reported that PAs were mostly accumulated in yolk, due to the 10-day yolk formation compared to 1-day egg white formation at a later stage and steady-state transfer of PAs into eggs (P. Mulder, de Witte, et al., 2016).
4.1.3.2. Hazard characterisation
PAs have a common toxicity profile with the liver being the main target organ of toxicity. IARC has classified three PAs, lasiocarpine, monocrotaline and riddelliine, as Group 2B, ‘possibly carcinogenic to humans’ and other PAs as Group 3, “not classifiable as to its carcinogenicity to humans” due to the limited information available (Codex, 2014; IARC, 1987).
EFSA concluded that 1,2-unsaturated PAs may act as genotoxic carcinogens in humans based on the available knowledge and assessed the sum of 1,2-unsaturated PAs together, assuming equal potency. A BMDL10 for excess cancer risk of 237 µg/kg bw/day for induction of liver haemangiosarcomas by riddelliine in female rats was calculated as the reference point for comparison with the estimated dietary exposure (EFSA, 2017a). EFSA recommended an MOE approach for cumulative chronic exposure levels of PAs, and determined that an MOE of 10,000 or higher, is of low concern from a public health perspective (EFSA, 2017a).
A study was conducted in Europe with a total of 1,105 samples collected. These comprised milk and milk products, eggs, meat and meat products, teas, and food supplements collected in supermarkets, retail shops, and via the internet. PAs were detected at 0.10–0.12 µg/kg PAs in 1% of egg samples (2 out of 205 egg samples) (P. P. J. Mulder, López, et al., 2018).
Reg. (EU) 2023/915 and assimilated Reg.(EU) 2020/2040 in GB has set MLs for 21 different PAs in food for plant origin (except pollen-based food supplements, pollen and pollen products) in a range of 1 – 1,000 μg/kg (European Commission, 2023a; Gov UK, 2020b). No MLs were defined for any food of animal origin, including eggs.
Directive 2002/32/EC specifies the MLs of contaminants in feed (European Parliament, 2002; Gov UK, 2002). No MLs were set specifically for PAs but Section VI specifies MLs for botanical impurities, such as weed seeds and unground and uncrushed fruits containing alkaloids, glucosides or other toxic substances separately or in combination (3,000 mg/kg).
4.1.4. Conclusion
Mycotoxins (aflatoxins, OTA) and PAs can contaminate feed and result in poultry exposure. Hot and humid climates are risk factors for aflatoxin and OTA formation in feed (EFSA, 2004a). PA concentrations in feed are expected to be higher when nutrient availability is low and increasing soil moisture might lead to higher PA-concentrations in the roots (Codex, 2014). Due to the metabolism and elimination of these agricultural contaminants by poultry, transfer from feed into eggs is limited. Generally experimental studies showed that, unless maximum levels in feed are exceeded, there is low concern for transfer into eggs. No MLs were set for mycotoxins or PAs in eggs.
There are concerns relating to genotoxicity and carcinogenicity for the specified mycotoxins and PAs, and they are generally assumed to have no safe threshold. Therefore, if aflatoxins, OTA or PAs are found to be present in eggs, there may be a concern for consumer health and further assessment would be required.
4.2. Biocides
The biocides that were shortlisted for characterisation were fipronil and chlorate. A conclusion section on biocides follows the individual hazard characterisation sections (4.2.14.2.2).
4.2.1. Fipronil
4.2.1.1. Hazard route
Due to its insecticidal properties, fipronil is also used as a veterinary medicine and a biocide. It is currently authorised as a veterinary medicine in GB and NI for the control of fleas in domestic animals, there are no approved uses on livestock, including chickens (VMD, 2024c). Fipronil is currently authorised as biocide in GB for use as an insecticide and acaricide (PT18) (HSE, 2024c). Residues of fipronil in eggs may also arise from the use of fipronil as an insecticidal pesticide, however it has not been approved as a pesticide in GB and the EU since 2018 (European Commission, 2016).
EFSA concluded that the residues of fipronil detected in eggs in the 2019 monitoring were as a result of illegal use as a biocide or veterinary medicine, rather than from pesticide usage (EFSA, 2021c). In 2017 there was a large scale incident in which fipronil was detected in EEPs in over 45 countries, due to the illegal use of fipronil as a mite treatment for chickens (European Commission, 2018b).
4.2.1.2. Hazard Characterisation
Fipronil is toxic by oral, inhalation and dermal acute exposure. Adverse effects in the central nervous system, liver and thyroid were identified in short term studies. EFSA set an Acceptable Daily Intake (ADI)of 0.0002 mg/kg bw/day, and an acute reference dose (ARfD) of 0.009 mg/kg bw (EFSA, 2006).
Appendix VIII summarises the levels of fipronil detected in the literature and alerts. Levels of fipronil in food are controlled via pesticide MRLs under Reg. (EU) 396/2005, with a MRL of 0.005* mg/kg in place for eggs, where * denotes the MRL is set at the limit of quantification (LOQ) (European Parliament, 2005; Gov UK, 2005).
4.2.2. Chlorate
4.2.2.1. Hazard Route
Chlorate is a by-product or residue of chlorine based disinfectant usage in drinking water, irrigation, food processing and veterinary hygiene, which may lead to residues in foods, including eggs. (FERA, 2017b). Chlorine dioxide is currently authorised as biocide in GB for use as an disinfectant (PT02-PT05) (HSE, 2024c). Residues of chlorate in eggs may arise from the use of the herbicide sodium chloride, however this pesticide has not been approved in GB and the EU since 2008 (FERA, 2017b).
4.2.2.2. Hazard Characterisation
Chronic exposure to chlorate can inhibit iodine uptake, potentially leading to iodine deficiency, EFSA have set a Tolerable Daily Intake (TDI) of 3 μg/kg bw/day. Acute exposure can limit oxygen absorption from the blood leading to kidney failure. EFSA set an ADI of 36 μg/kg bw/day derived from a No Observed Adverse Effect Level (NOAEL) (EFSA, 2015a).
Appendix VIII summarises the levels of chlorate detected in the literature and alerts. Levels of chlorate in food are controlled via pesticide MRLs under Reg. (EU) 396/2005, with a MRL of 0.05 mg/kg in place for eggs (European Parliament, 2005; Gov UK, 2005). Chlorate MRLs are set at a level which accounts for the potential for food to come into contact with chlorate residues via routes such as drinking water or processing, and that contribution to the measured level from these sources should be taken into account (Reg. (EU) 2020/749) (European Commision, 2020; Gov UK, 2020a).
4.2.3. Conclusion
Cases of non-compliance of fipronil and chlorate have been detected in eggs (when compared to MRLs for these substances set under pesticide regulations) which may arise from their use as biocides. There are potential adverse effects which may result from exposure to residues of these substances. The presence of residues of these chemicals in eggs at levels exceeding the MRL, may require further action in the form of a risk assessment to determine the risk to consumer.
4.3. Environmental Contaminants
The environmental contaminants shortlisted for characterisation are dioxins and dioxin-like substances, PCBs, PFAS, PCNs and melamine. Conclusions on the concerns regarding the potential environmental contaminant presence in EEPs are presented in section 4.3.5.
4.3.1. Dioxins, dioxin-like substances and PCBs
4.3.1.1. Hazard route
Dioxins are a group of 75 PCDD and 135 PCDF related chemicals that occur naturally and are widely present in the environment, although mainly as unwanted byproducts of combustion and of various industrial processes (COT, 2010).
PCBs are not natural substances; they were globally manufactured and used until prohibition of manufacture in the late 1980s (EFSA, 2010a; WHO, 2019). PCBs can contaminate the environment from materials (e.g., paint) or waste sites containing PCBs (EFSA, 2010a). PCBs can be divided into dioxin like PCBs (DL-PCBs) which exhibit similar biological activity to dioxins, and non-dioxin like PCBs (NDL-PCBs).
PCBs, PCDDs and PCDFs are listed under the Stockholm Convention, as POPs (Stockholm Convention, 2019).
When released into the air, dioxins and DL-PCBs are deposited on plants and on soil, consequently contaminating both food and feed (Codex, 2018). Through transfer from animal feed, dioxins and DL-PCBs accumulate in tissues and can be excreted in fat-containing products such as milk and eggs (Codex, 2018; RIVM, 2019). In laying hens, dioxins and DL-PCBs may concentrate in the fat content of the egg yolk (Codex, 2018).
EFSA reported that the highest mean contamination level of NDL-PCBs was observed in fish and fish derived products followed by eggs in an analysis conducted from samples in northern Europe (EFSA, 2010a).
4.3.1.2. Hazard characterisation
Dioxins and DL-PCBs
IARC classified PCDDs, PCDFs and DL-PCBs as Group 1: Carcinogenic to humans (IARC, 1997, 2015). COT assessed dioxins and DL-PCBs in 2001 and concluded that the health effects most likely to be associated with low levels of exposure relate to the developing embryo/foetus and concluded that there is the potential for a range of adverse health effects. The COT proposed a TDI of 2 pg WHO-TEQ/kg bw/day based upon effects on the developing male reproductive system mediated via the maternal body burden. They considered this to be adequate to protect against other possible effects such as cancer and cardiovascular effects (COT, 2001).
EFSA re-evaluated dioxins and DL-PCBs in 2018 and proposed a reduction to the tolerable weekly intake (TWI), to 2 pg TEQ/kg bw (EFSA, 2018a). The COT performed a further assessment following the EFSA update and stated that while the re-assessment of dioxins is a necessary and important piece of work going forward, further review of dioxins will be an extensive and lengthy undertaking. In the meantime, COT has concluded it was not necessary to update their existing advice at this point and maintained current TDI of 2 pg/kg bw per day (COT, 2021b).
A survey by EFSA (2012) investigated the presence of the sum of dioxin and DL-PCBs in hen eggs and egg products, and found their presence with a maximum value of 11.96 pg WHO-TEQ/g (EFSA, 2012c). A summary of the levels of dioxin and DL-PCBs in EEPs identified in the literature are given in Appendix VI, with dioxins and DL-PCBs detected at levels up to 249.1 pg WHO-PCDD/F-PCB-TEQ/g fat (Hoang, Traag, et al., 2014).
Levels of dioxins and dioxin-like PCBs are controlled in EEPs in GB and NI in assimilated Reg. (EC) 1881/2006 and Reg. (EC) 2023/915 respectively. The MLs are 2.5 pg/g fat for the sum of dioxins (WHO-PCDD/F-TEQ) and 5.0 pg/g fat for the sum of dioxins and DL-PCBs (WHO-PCDD/F-PCB- TEQ) (European Commission, 2023a; Gov UK, 2006).
NDL-PCBs
NDL-PCBs are reported as the sum of six PCB congeners (PCB 28, 52, 101, 138, 153, 180) as they represent approximately 50% of the total NDL-PCBs found in food and relevant degrees of chlorination (EFSA, 2010a). The German Federal Institute for Risk Assessment (BfR) assessed NDL-PCBs and found that NDL-PCB mixtures only have a low potential for acute toxicity (BfR, 2018b). However, the liver and the thyroid have been identified as the most sensitive target organs in animal experiments after long(er) term exposures with individual NDL-PCB congeners. A HBGV for NDL-PCBs has not been established due to the insufficient toxicity data available. There is limited evidence in experimental animals for the carcinogenicity of some NDL-PCBs (IARC, 2015).
A survey by EFSA in 2010 found that NDL-PCBs were present in hen eggs and egg products at up to 16.7 µg/kg. Eggs were the second most contaminated food product category and 94% of hen eggs and hen egg product samples had NDL-PCB levels above the LOQ (EFSA, 2010a). A summary of the levels of NDL-PCBs in EEPs identified in the literature are given in Appendix VI, with NDL-PCBs detected at levels up to 218 ng/g fat (Squadrone, Brizio, et al., 2015).
Levels of NDL-PCBs are controlled in EEPs in GB and NI in assimilated Reg. (EC) 1881/2006 and Reg. (EC) 2023/915 respectively. The ML is 40 ng/g fat for the sum of NDL-PCBs. (European Commission, 2023a; Gov UK, 2006).
4.3.2. Melamine
4.3.2.1. Hazard route
Melamine is used mainly in the synthesis of melamine–formaldehyde resins for the manufacture of plastics, coatings, adhesives, and moulding compounds (dishware and kitchenware) (WHO, 2008).
Melamine can be present in food as a result of uses in food contact materials (FCMs), including articles made of melamine or melamine-formaldehyde plastics, coatings (EFSA, 2010c), adhesives or bamboo FCMs (COT, 2024b). Melamine may also enter the food chain indirectly from trichloromelamine which is used in sanitising solutions for food-processing equipment and food-contact articles (Codex, 2010).
Melamine in food or feed can be found at “baseline” levels, i.e., levels occurring indirectly from the approved uses of melamine or melamine precursors (Codex, 2010). It can also be found at “adulteration” levels, which refer to levels that result from the intentional, illegal addition of melamine or melamine–precursors directly to food and/or feed (Codex, 2010). Some melamine levels in eggs have been attributed to carry-over from adulterated animal feed (WHO, 2008). The deliberate addition of melamine to food is not permitted in Europe, the USA or in the UK.
Melamine can form as a metabolite from triazine-based pesticides/herbicides, in particular cyromazine (Codex, 2010). Cyromazine can be also used as a veterinary medicine in some countries, and melamine might be present as an impurity in feed additives (e.g., Guanidino acetic acid (GAA) can contain up to 15 mg/kg melamine and up to 25 mg/kg of melamine and structurally related compounds) (Codex, 2010). As such, animal feed may contain melamine and result in carryover into products of animal origin including eggs (Codex, 2010).
4.3.2.2. Hazard characterisation
Melamine does not exhibit systemic toxicity, but is able to complex with other substances such as endogenous uric acid or substances related to melamine to form crystals in the urine, which cause kidney damage (EFSA, 2010c). Illegal adulteration of food and feed with melamine has resulted in illnesses and deaths of human infants, primarily as a result of kidney damage caused by crystals or stones in the urinary tract (EFSA, 2010c). In late 2008, approximately 300,000 infants in China were affected by infant formula containing melamine, including six confirmed deaths (EFSA, 2010c). Clinical signs included vomiting, fever, haematuria, dysuria, oliguria, anuria, high blood pressure, oedema and pain in kidney areas (WHO, 2008).
EFSA established a TDI of 0.2 mg/kg bw/day melamine derived from a BMDL10 of 19 mg/kg bw/day (and by applying UF of 100) based on urinary bladder crystals observed in male rats, which was considered as adequate for the protection of infants (EFSA, 2010c). The same TDI was derived by the World Health Organisation (WHO) (WHO, 2008). Based on experimentally spiked feed (10 mg/kg melamine), transfer rates of melamine to eggs were calculated between 1.5% – 3.2% (EFSA, 2010c).
A summary of the levels of melamine in EEPs identified in the literature are given in Appendix VI, with melamine detected at levels up to 1.98 mg/kg in whole fresh egg (Shakerian, Khamesipour, et al., 2018).
Levels of melamine are controlled in EEPs in GB and NI in assimilated Reg. (EC) 1881/2006 and Reg. (EC) 2023/915 respectively. with a maximum level of 2.5 mg/kg in food, except where “the maximum level does not apply to food for which it can be proven that the level of melamine higher than 2.5 mg/kg is the consequence of authorized use of cyromazine as insecticide. The melamine level shall not exceed the level of cyromazine” (European Commission, 2023a; Gov UK, 2006).
Reg. (EU) 2017/2229 in EU and assimilated Reg. (EU) 2017/2229 in GB specifies the maximum content for melamine as 2.5 mg/kg for feed, except if feed contains the GAA additive, which is specified to contain melamine as impurity up to 20 mg/kg (European Commission, 2017; Gov UK, 2017). However, this is a specified use only for chickens for fattening but not laying hens.
4.3.3. Polychlorinated naphthalenes (PCNs)
4.3.3.1. Hazard route
The main source of PCNs is through industrial production and as a by-product of other industrial processes. PCNs are widely present in the environment and have been shown to be highly bio-accumulative. Available data in feed and food show widespread occurrence in both (EFSA, 2024f). The most important exposure route of the general population is suggested to occur orally via foodstuffs (CHM, 2013).
Studies have shown that routes of exposure to PCNs for poultry are likely be via contaminated feed (C. Wang et al., 2022), or potentially through bedding materials in chicken housing (Fernandes, Lake, et al., 2023).
4.3.3.2. Hazard characterisation
Exposure to PCNs leads to several adverse effects including hepatotoxicity, neurotoxicity and immune response suppression along with endocrine disruption, leading to reproductive disorders and embryotoxicity (Fernandes, Kilanowicz, et al., 2022).
PCNs have similar mechanisms of toxicity to dioxin-like compounds (CHM, 2013; EFSA, 2024f). A number of short and medium term studies prove high short-term toxicity at relatively low concentrations (>3mg/kg) (CHM, 2013).
EFSA’s risk assessment on PCNs in feed and food focused on hexa chlorinated napthalenes (hexaCNs) due to limited data on other PCN congeners. The haematological system, liver and thymus were identified as main targets for hexaCNs by EFSA as well as developmental effects (EFSA, 2024f). EFSA concluded that due to limitations in the data, the derivation of a HBGV was not appropriate and an MOE approach should be applied. EFSA proposed a BMDL20 of 0.05 mg/kg bw/day based on a considerable decrease in the platelet count observed in rats. They considered that MOEs ≥ 2000 are sufficient to conclude that the current dietary exposure to hexaCNs does not raise a health concern (EFSA, 2024f).
A survey carried out by EFSA found that among the food categories in which hexaCNs contamination would be expected, the highest percentage of quantified data was found in the “eggs and egg products” category, with the highest mean concentration of 5.18 ng/kg (upper bound) reported for “whole eggs” (EFSA, 2024f). EFSA suggested this is due to the lipophilicy of PCNs and their tendency to concentrate in fatty foods (EFSA, 2024f).
A summary of the levels of PCNs in EEPs identified in the literature are given in Appendix VI, with PCNs detected at levels up to 20 ng/kg whole weight with the highest levels occurring in duck, goose and gull eggs (FERA, 2017a).
PCNs are not currently regulated under any specific GB or EU regulation for food or feed.
4.3.4. Polyfluorinated substances (PFAS)
4.3.4.1. Hazard route
PFAS are used in industrial applications and for production of consumer products. Many PFAS are environmentally long-lived and individuals are exposed to them through all environmental sources (COT, 2022b). A number of PFAS compounds are relatively easily soluble in water, therefore these compounds can easily spread via water and aerosols in the environment (BuRO, 2024). Food can become contaminated through contact with soil and water, via uptake through animal feed and water, through food packaging containing PFAS, or contact with processing equipment that contains PFAS (EFSA, 2020a).
In food, perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA) and their salts are found at the highest concentrations, compared to other PFAS types (European Commission, 2024c). PFAS may occur in eggs if the chickens ingest feed, water or soil contaminated with PFAS (BuRO, 2024). A study investigated the transfer of environmental contaminants including PFAS into hen eggs via poultry bedding. The highest PFAS contaminated bedding materials were shredded cardboard and dried paper sludge, and there was evidence for uptake of PFOS, and PFOA in chickens from this bedding (Fernandes, Lake, et al., 2023).
4.3.4.2. Hazard characterisation
In 2020 EFSA concluded that based on available studies in animals and humans, effects on the immune system were considered the most critical effect and equal potencies were assumed for the four PFAS assessed (PFOA, PFOS, perfluorononanoic acid (PFNA) and perfluorohexanesulphonic acid (PFHxS)) (EFSA, 2020e). A TWI of 4.4 ng/kg bw per week (0.63 ng/kg bw/day) was established from a BMDL10 of 17.5 ng/mL for the sum of the four PFAS in serum based on epidemiological studies (EFSA, 2020e).
EFSA’s approach was reviewed by the COT who raised concerns (e.g., uncertainties with the critical endpoint used and reservations with some of the modelling). The COT are currently conducting their own extensive review of PFAS. In the meantime, the COT has advised that where risk assessments are undertaken for PFAS, consideration should be made of the various HBGVs established by different authoritative bodies for the specific compounds identified, recognising the uncertainties regarding the critical effects they are based on, and the modelling approaches used (COT, 2022b).
Eggs were identified as one of the major contributors for PFAS in the diet, specifically for PFOS and PFOA (EFSA, 2020e). In 2020 EFSA estimated that eggs and egg products contributed up to 41% (mean, lower bound) of PFOS for infants’ exposure and up to 40% (mean, lower bound) of PFOA for adults’ exposure (EFSA, 2020e).
A summary of the levels of PFASs in EEPs identified in the literature are given in Appendix VI, with PFOA detected at levels up to 3.14 µg/kg in duck eggs (Qi, Zhou, et al., 2019).
Levels of PFAS are controlled in eggs in NI (EU 2023/915), with maximum levels of 1.0 μg/kg (PFOS), 0.3 μg/kg (PFOA), 0.7 μg/kg (PFNA), 0.3 μg/kg (PFHxS), 1.7 μg/kg (sum of PFOS, PFOA, PFNA, PFHxS) (European Commission, 2023a; Gov UK, 2006).
4.3.5. Conclusion
With the exception of melamine, the identified environmental contaminants included in this section are POPs, listed under the Stockholm Convention (Stockholm Convention, 2019). POPs become widely distributed throughout the environment (soil, water and air), they bioaccumulate and they are found at higher concentrations at higher levels in the food chain (Stockholm Convention, 2019). Therefore, exposure of poultry to POPs can occur through water, feed, soil and also from bedding materials used in husbandry and transferred into the eggs (Fernandes, Lake, et al., 2023).
For melamine, contamination in eggs may arise due to multiple pathways such from its pre-cursors e.g. GAA: and cyromazine or via its use in FCMs. Melamine is also associated with illegal adulteration in food and feed due to its ability to artificially increase the apparent protein content (Codex, 2010).
Toxicological effects of environmental contaminants vary and therefore further assessment would be required to determine whether a specific environmental contaminant detected in EEPs at a specific level, would be a risk to consumers.
4.4. Metals
The metals shortlisted for characterisation are arsenic, cadmium, chromium, copper, mercury, lead and selenium. Conclusions on the concerns regarding the potential metal presence in EEPs are presented in section 4.4.8.
4.4.1. Arsenic
4.4.1.1. Hazard route
Arsenic, in different inorganic and organic forms, can be present in the environment naturally, as well as anthropogenically through practices such as farming and industrial pollution (EFSA, 2009b). Inorganic arsenic in food and feed are generally found in the +3 or +5 oxidation state, present as thio complexes or as the oxo anions, arsenite and arsenate (EFSA, 2023g). Dimethylarsinic acid (DMA(V)) is by far the most abundant of small organic arsenic species in food (EFSA, 2024e).
Drinking water is one of the most significant sources of exposure to arsenic. Inorganic arsenic is naturally present at high levels in the groundwater of a number of countries (WHO, 2022a). Crops irrigated with contaminated water and food prepared with contaminated water are the main sources of arsenic exposure (EFSA, 2009b; WHO, 2022a).
The main contributors to overall dietary exposure to inorganic arsenic were considered to be rice, grains and drinking water (EFSA, 2023g). Inorganic arsenic is the predominant form found in meats, poultry, dairy products and cereal (IARC, 2012).
4.4.1.2. Hazard characterisation
Arsenic toxicity depends on its molecular form as inorganic arsenic is more toxic than the organic species. Toxicity of inorganic arsenic is well characterised, whilst there is less information available for organic arsenic.
EFSA has published a risk assessment of small organoarsenic species in food (EFSA, 2024e) which focussed on dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA) (EFSA, 2024e) for which toxicological information is available.
With respect to inorganic arsenic, acute and subacute exposure can affect almost all physiological systems of the body (EFSA, 2023g). The health outcomes associated with chronic ingestion of inorganic arsenic include skin, bladder and lung cancer, skin lesions, developmental and neurodevelopmental effects, heart diseases, respiratory and kidney diseases, spontaneous abortion, stillbirth and infant mortality (EFSA, 2023g). “Arsenic and inorganic arsenic compounds” are classified as Group 1 carcinogens by IARC: carcinogenic to humans (IARC, 2012). Dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA) acid are classified as Group 2B carcinogens by IARC: possibly carcinogenic to humans (IARC, 2012).
Inorganic arsenic is a genotoxic carcinogen and so no health based guidance values can be set (EFSA, 2023g; IARC, 2012). The COT noted that inorganic arsenic was an indirect genotoxin, which would have a threshold. COT recommended that risk assessment is performed against the JECFA BMDL0.5 of 3.0 μg/kg bw/day based on increased incidence of lung cancer. Considering the mechanistic data of genotoxicity by inorganic arsenic, COT concluded that a MOE of 10 was considered an appropriate level of concern (COT, 2023).
For small organic arsenic species, due to the incomplete toxicological data, EFSA has applied an MOE approach. EFSA has established a BMDL10 of 18.2 mg/kg /bw/day for MMA(V) based on the gastrointestinal effects of diarrhoea in rats. An MOE of ≥ 500 was identified not to raise a health concern. A BMDL10 of 1.1 mg/kg /bw/day for DMA(V) was established based on an increased incidence of urinary bladder tumours in rats. For DMA(V), an MOE of ≥10,000 was identified as of low health concern as EFSA considered DMA(V) to be genotoxic and carcinogenic. The mechanisms of genotoxicity and its role in carcinogenicity have not been fully elucidated although potential direct and indirect mechanisms have been suggested (EFSA, 2024e).
Occurrence data on inorganic arsenic by EFSA, showed that inorganic and organic arsenic levels in eggs and egg products were left-censored data (i.e., below the limit of detection [LOD] or limit of quantification [LOQ). Eggs were not a primary contributor to inorganic arsenic exposure from the data obtained from EU countries (EFSA, 2021a, 2023g, 2024e). The LOQ in EFSA’s assessment was 0.01 mg/kg for inorganic arsenic (EFSA, 2021a, 2023g). No LOQ was reported for organic arsenic species (EFSA, 2024e).
In contrast to the EFSA data, arsenic levels in eggs reported in the literature were above the LOQ and are summarised in Appendix VII, with levels of up to 0.049 mg/kg detected in whole eggs (Chernikova, Pityurina, et al., 2020).
No ML of arsenic in eggs is established in GB or NI. However, MLs of inorganic arsenic for other commodities are between 0.01 – 0.25 mg/kg in Reg. (EU) 2023/915 (European Commission, 2023a) and between 0.10 – 0.3 mg/kg in assimilated Reg. (EU) 2015/1006 (Gov UK, 2015b).
4.4.2. Cadmium
4.4.2.1. Hazard route
Cadmium exposure is through food, water and air, however food is understood to be the main source of human exposure (COT, 2024a). The presence of heavy metals, including cadmium, in eggs is likely to result from industry and agriculture. This includes use of contaminated pesticides and fertilisers and irrigation of crops with contaminated water (Aljohani, 2023). Cadmium levels from the samples analysed in the EU showed that eggs and egg products were the food category that contributed the least to the dietary exposure (EFSA, 2012a). However, the literature review indicated that cadmium has been detected in eggs and eggs products (see Section 4.4.2.2 for further detail).
4.4.2.2. Hazard characterisation
Chronic ingestion of cadmium has been shown in experimental animals to result in a wide range of health effects including metabolic disorders, nephrotoxicity and hepatotoxicity as well as adverse effects for pregnant women and unborn babies (COT, 2022c). Cadmium and cadmium compounds are classified as a Group 1 Carcinogen: Carcinogenic to humans by IARC (IARC, 2012). A report by the European Chemicals Bureau stated that there is no evidence to show that cadmium causes cancer through the oral route of exposure (JRC, 2008).
The critical adverse effect for cadmium is kidney damage. Therefore, JECFA and EFSA have derived a TWI of 25 µg/kg bw/week based on changes in kidney function, for the protection of possible kidney damage later in life (EFSA, 2009a; JECFA, 2007).
Cadmium levels in eggs reported in the literature are summarised in Appendix VII, with levels of up to 0.013 mg/kg detected in whole eggs (Salar-Amoli & Ali-Esfahani, 2015).
There are no regulatory levels for cadmium in eggs, however the MLs for other food groups (plant originated) range between 0.01 – 1 mg/kg (poultry meat: 0.05 mg/kg) in Reg. (EC) 2023/915 in EU and in assimilated Reg. (EC) No 1881/2006 (European Commission, 2023a; Gov UK, 2006).
4.4.3. Chromium
4.4.3.1. Hazard route
Chromium can enter the food chain via the different environmental pathways (e.g., water or soil), either because of its natural presence or emission from anthropogenic activities. It is used in a wide variety of processes including alloy production, and is present in a wide range of products such as; ceramics and glass, fungicides, pigments and especially in stainless steel (COT, 2018a).
The presence of heavy metals including chromium in eggs is likely to result from industry and agriculture, including use of contaminated pesticides and fertilisers, irrigation of crops with contaminated water (Aljohani, 2023).
Food preparation with chromium containing materials (e.g., processors, utensils) could represent an additional source for the presence of chromium in food. Exposure to the more toxic Cr(VI) is primarily through drinking water, although some exposure occurs through food (EFSA, 2014d).
4.4.3.2. Hazard characterisation
Chromium is most commonly found in two oxidation states, either chromium (III) (Cr(III)) or chromium (VI) (Cr(VI)). The most prevalent natural form of chromium is Cr(III) and food is considered to be a source of Cr(III) which is an essential micronutrient (COT, 2018a). The toxicity of chromium is dependent on the speciation of chromium. Although long term respiratory exposure to Cr(VI) can cause lung cancer, the IARC has concluded that there is not sufficient evidence to support that Cr(VI) ingested via food and water is a carcinogen. Both Cr(III) and (VI) can cause other non-carcinogenic chronic effects on the liver and kidney and in the blood (COT, 2018a).
EFSA established a TDI for Cr(III) of 300 µg/kg bw/day, based on the NOAEL from sub-chronic and long-term toxicity in rats. Based on their assessments, EFSA concluded that dietary exposure to Cr(III) would be considered unlikely to result in cancer in humans (EFSA, 2014b).
Regarding Cr(VI), for carcinogenic effects, ESFA derived a BMDL10 of 1.0 mg/kg bw/day for combined adenomas and carcinomas observed in the small intestines of male and female mice, with an MOE of ≥10,000 being of low concern (EFSA, 2014d). For non-carcinogenic effects EFSA have determined an MOE of 100, which would indicate a low concern for human health and derived:
-
BMDL10 value of 0.11 mg/kg bw/day for adverse effects in duodenum and,
-
BMDL05 of 0.2 mg/kg bw/day for adverse haematological effects
In a 2014 review, EFSA determined the mean concentration of chromium from eggs as 22 µg/kg (LB) and 29 (UB) µg/kg (EFSA, 2014d). Levels of chromium in egg and egg products, were mostly lower than other food or drink categories (EFSA, 2014d).
In contrast to the EFSA data, chromium levels in eggs reported in the literature are summarised in Appendix VII, with levels of up to 0.24 mg/kg detected in whole eggs (Salar-Amoli & Ali-Esfahani, 2015).
There are no regulatory levels for chromium in food, however the WHO have derived a guideline value of 0.05 mg/L in water (WHO, 2020b).
4.4.4. Copper
4.4.4.1. Hazard route
Copper can be used as a PPP and fertiliser, and as a result it can also contribute to the concentration of copper in soil (EFSA, 2023b). Due to the homeostatic control of copper uptake from soil by plants, short-term applications of copper to plants may have limited impact on the detected levels. However in the longer-term, due to increasing concentrations in soil over time, there is likely to be increased copper uptake by crops (EFSA, 2023b). Additionally, copper can be present in drinking water due to leaching from copper plumbing (FWR, 2017). Copper can be also used as a feed additive in poultry (EU 2018/1039; EU 2022/1459) (EFSA, 2016b; European Commission, 2018a, 2022a). As a result of, concentrations from crops (when used as a feed) and the use as an additive in poultry feed, copper is expected to be present in the eggs.
A study by Kabeer et al., sought to investigate the relationship between heavy metal contamination in feed, water and eggs. Researchers concluded that, increased heavy metals levels in eggs, including copper was a result of exposure of poultry to contaminated feed and water (Kabeer, Hameed, et al., 2021).
4.4.4.2. Hazard characterisation
Copper has a tight homeostatic regulation in the body, which is critical for the prevention of copper toxicity. The development of chronic copper toxicity is dependent on copper homeostasis and its tissue retention (EFSA, 2022b).
Adverse health effects at higher levels include an increase in systolic blood pressure and impaired metabolism of other nutrients. At high excess, there are irreversible metabolic changes and in a worst case can result in death (COT, 2018c). The most critical effect of copper toxicity is liver injury due to accumulation. Copper retention is the critical pathway leading to toxicity and if intakes are not reduced, copper accumulation will occur in the liver resulting in hepatoxicity and extrahepatic toxicity (COT, 2018c; EFSA, 2022b). Based on the liver retention of copper, EFSA in 2022 established a copper Upper intake Level (UL) of 5 mg/day (at which no retention is expected) and an ADI of 0.07 mg/kg bw (EFSA, 2022b).
Eggs and egg products contribute 1–11% of the dietary copper intake of European populations (EFSA, 2016b).
Copper levels in eggs reported in the literature and alerts are summarised in Appendix VII, with levels of up to 8.5 mg/kg detected in whole duck eggs (Salar-Amoli & Ali-Esfahani, 2015).
Regulatory levels for copper in food are set under assimilated Reg. (EC) 396/2005 (European Parliament, 2005; Gov UK, 2005); the MRL for copper compounds in eggs is 2 mg/kg, based on their use as pesticides. The maximum content of copper in complete feed additives is 25 mg/kg for poultry. This maximum content includes copper already present in feed (e.g. due to natural occurrence or use of pesticides) and copper added as feed additive to meet the nutritional requirements of the animals (assimilated Reg. (EU) 2018/1039) (European Commission, 2018a; Gov UK, 2018).
4.4.5. Lead
4.4.5.1. Hazard route
Lead is an environmental contaminant which occurs both naturally and through human activities such as mining, smelting, etc. (EFSA, 2010b; WHO, 2023b). Drinking water delivered through lead pipes or pipes joined with lead solder may contain lead. However, measures have been taken to regulate levels of lead in pipes in Europe since the 1970s, which had a considerable effect in reducing exposure (EFSA, 2010b).
Farm animals including poultry, can be exposed to lead through feed and water contamination, and also from contaminated soil (FSA/VLA, 2024). For instance, in a case study, higher concentration of lead was reported in eggs collected from a farm close to mining area compared to commercially available eggs (Sartorius, Johnson, et al., 2022). Another study reported higher levels of lead in chickens that were exposed to lead-based paint in their environment (Trampel, Imerman, et al., 2003).
EFSA calculated the relative lead contribution of different food groups to total lead exposure. They considered individual consumption figures of food groups and mean lead concentrations in food groups. EFSA considered cereals, vegetables and tap water to contribute most to dietary exposure to lead for most European countries (EFSA, 2010b). The relative contribution of lead exposure from eggs, compared to the overall lead exposure (lower bound) was estimated as 0.13 in the GB and the highest contribution was observed in Poland (0.23). Lead levels in 11.4% of 615 analysed egg samples were above the LOD (EFSA, 2010b).
4.4.5.2. Hazard characterisation
The toxicity of lead differs according to whether it is in organic or inorganic form; organic lead is more toxic than inorganic lead (ASTDR, 2023). However, the dominant environmental exposure is to inorganic lead, while exposure to organic lead has predominantly been via occupational settings. Public Health England (PHE) (now the UK Health Security Agency) states that lead is a non-threshold contaminant and therefore may cause adverse health effects following exposure to any concentration (PHE, 2021). Exposure can lead to a wide range of serious adverse health effects. The most typical early symptom of lead poisoning, especially in occupational exposure or other high-level intakes of lead is colic (EFSA, 2010b). The most prominent symptoms are abdominal pain, nausea, vomiting and anorexia (EFSA, 2010b). However, owing to accumulation of lead in the body, adverse effects can occur from long-term dietary exposure at lower levels than would cause acute toxicity (EFSA, 2010b). The most critical chronic effects are developmental neurotoxicity in children/unborn fetus; cardiovascular effects and nephrotoxicity in adults (EFSA, 2010b; PHE, 2021). In children, absorption of lead is considered higher and they appear to be more sensitive than adults (EFSA, 2010b).
Developmental neurotoxicity resulting from exposure to lead is often assessed by decreased general intelligence (IQ) and the COT were not able to conclude on a threshold for exposure to lead below which developmental neurotoxicity was not observed (FSA, 2009b). For risk assessment of children, the COT concluded that the EFSA BMDL01 of 0.5 µg/kg bw/day (associated with a 1-point decrement in IQ) should be used. For adults, EFSA established BMDL10 of 0.63 mg/kg bw/d for nephrotoxicity and BMDL01 of 1.50 µg/kg bw/day for cardiovascular effects (EFSA, 2010b). In all cases an MOE of >1 was an indication that any risk from this exposure is likely to be small (but not such that it could be dismissed as of no potential concern), with an MOE of >10 being sufficient to ensure no appreciable risk. Because toxicity depends on total exposure to lead from all sources, it is important to consider combined exposures from food, water, and non-dietary sources (EFSA, 2010b).
Levels of lead of up to 0.21 mg/kg have been detected in eggs as reported in the EFSA review (EFSA, 2010b). Lead levels in eggs reported in the literature are summarised in Appendix VII, with levels of up to 0.35 mg/kg detected in whole eggs (Salar-Amoli & Ali-Esfahani, 2015).
There is no ML for lead in eggs in assimilated Reg. (EC) No. 1881/2006 and Reg. (EC) 2023/915 (European Commission, 2023a; Gov UK, 2006). However, the ML for lead in poultry meat (excluding offal) is set at 0.10 mg/kg. Noting the exposure to lead from different sources and the absence of a threshold for adverse health outcomes, any exposure to lead from eggs will add to cumulative exposure and is therefore undesirable.
4.4.6. Mercury
4.4.6.1. Hazard route
Mercury is an environmental contaminant which occurs both naturally and from anthropogenic sources. After release into the environment, it undergoes complex transformations and cycles between atmosphere, land and aquatic systems (European Commission; COT, 2018b; EFSA, 2012b). Mercury can be found in three chemical forms: (1) elemental or metallic mercury (Hg(0)), (2) inorganic mercury (mercurous (Hg22+) and mercuric (Hg2+) cations) and (3) organic mercury (e.g., methylmercury) (EFSA, 2012b).
Methylmercury is the most common form in the food chain and exposure to methyl mercury occurs mostly via diet (European Commission; EFSA, 2012b). Mercury can be also found in feeding stuffs (EFSA, 2008b). Levels of mercury reported in feeding stuffs were reported to EFSA above the ML of 0.1 mg/kg, mostly for “unspecified feeds and raw materials” (10.9% occurrence) during 2002 – 2006 (EFSA, 2012b). Average and maximum concentrations of total mercury in complete feeding stuffs for poultry were below the ML during these years (EFSA, 2012b). Mercury (as total) was detected in eggs, ranging from 0.6 – 6.3 μg/kg (mean LB and P95, respectively) (EFSA, 2012b). The literature review indicated that mercury has been detected in eggs (see Section 4.4.6.2 for further detail).
4.4.6.2. Hazard characterisation
The toxicity of mercury differs according to whether it is in organic, inorganic, or metallic form. The forms of mercury differ in their effects on the nervous, digestive and immune systems, and on lungs, kidneys, skin and eyes (WHO, 2017a). Organic mercury, particularly methylmercury, is the form more extensively absorbed following ingestion, and can cross the blood-brain barrier and the placenta. This can cause effects on neurodevelopment in the embryo or in young children (COT, 2018b). In their risk assessment, the COT concluded that the EFSA HBGV was appropriate: a TWI for methylmercury of 1.3 μg/kg bw/week (expressed as mercury), which is based on neurodevelopmental adverse effects after prenatal exposure (EFSA, 2012b). In 2012, EFSA adopted the provisional TWI (PTWI)of 4 μg/kg bw/week established by JECFA in 2010, for inorganic mercury, based on an increase in relative kidney weight observed in rats (EFSA, 2012b; JECFA, 2010).
Mercury levels in eggs reported in the literature are summarised in Appendix VII, with levels of up to 17.47 mg/kg detected in whole eggs (Aendo, Garine-Wichatitsky, et al., 2022).
There is no ML for mercury in eggs in assimilated Reg. (EC) No. 1881/2006 and Reg. (EC) 2023/915 (European Commission, 2023a; Gov UK, 2006). However, as exposure to mercury can come from different sources, any additional exposure to mercury will add to cumulative exposure and is therefore undesirable.
4.4.7. Selenium
4.4.7.1. Hazard route
Selenium can enter the food chain via the different environmental compartments (e.g. soil, water), either because of natural presence or emission from anthropogenic activities. It is an essential micronutrient, and food can be enriched with selenium. (EFSA, 2022d). Selenium can be also used as a fertiliser and as a feed additive for poultry (EU 2022/1459) (European Commission, 2022a). Taken altogether, concentrations from crops and being used as an additive in poultry, selenium is expected to be distributed into eggs.
4.4.7.2. Hazard characterisation
In the diet, selenium is mainly present in organic compounds, as L-selenomethionine and L-selenocysteine, with lower amounts in the inorganic form (e.g., selenate) (EFSA, 2022d). High intakes of selenium will result in selenosis (selenium poisoning) (EFSA, 2022d). Acute selenosis is characterised by symptoms including hypotension and tachycardia, gastrointestinal effects, pulmonary oedema, neurologic abnormalities, delirium and coma. Chronic selenosis includes symptoms such as brittle nails, brittle hair, alopecia, neurological abnormalities and decreased cognitive function (EFSA, 2022d).
An UL of 255 μg/day (3.64 μg/kg bw/day) for adults was derived from a lowest-observed-adverse-effect-level (LOAEL) of 330 μg/day based on alopecia (men, ≥ 50 years), an early sign of selenium toxicity. In 2022, EFSA highlighted that based on available intake data, adult consumers are unlikely to exceed the selenium UL of 255 μg/day, except for regular users of food supplements containing high daily doses of selenium or regular consumers of Brazil nuts (EFSA, 2022d). ULs of selenium in infants, children and adolescents were based on the UL of adults, adjusted for the body weight, as EFSA reports that there is no indication from the literature that children may be more susceptible than adults to selenium toxicity (EFSA, 2022d).
In 2024, EFSA reported that where poultry were fed at the maximum authorised level in animal feed, their eggs were identified to be a relatively high POAO source of selenium (EFSA, 2024c). Levels of 0.327 mg/kg fresh matter (FM) of inorganic selenium and 0.366 mg/kg FM of organic selenium were measured in eggs, exceeded only by liver and offal of all species. Based on the exposure assessment from food of animal origin, EFSA concluded that when complete feeds were supplemented at about 0.2 mg/kg feed (with total selenium ≤ 0.5 mg/kg feed), the adult UL was met or exceeded for all population groups (except elderly and very elderly), suggesting a potential concern for consumer safety.
There are no MLs for selenium in food and feed, however selenium is regulated in complete animal feed with a maximum content of 0.5 mg/kg in the EU (NI) under Reg. (EU) 2022/1459 (European Commission, 2022a).
4.4.8. Conclusion
A range of metals may contaminate eggs, and dietary exposure to metals, in particular heavy metals, may be a human health concern. As there are multiple sources of exposure to heavy metals due to their wide occurrence in environment and food, any significant additional exposures, for example from contaminated eggs, is undesirable as it could contribute to the overall background exposure in the UK population.
The toxicity of different metals varies, and they have been detected in eggs at varying concentrations. Therefore, a risk assessment would be required to determine the risk to consumers from contamination of EEPs with a specific metal at a particular concentration, especially those metals which have no threshold regarding safety, due to their high toxicity (e.g., lead, arsenic). No MLs for heavy metals are in place for eggs, however a number of heavy metals have been reported in eggs above the LOQ and at levels that would exceed relevant regulatory levels in other similar commodities where they are set. Although this should not be taken to confirm a risk from eggs without further assessment.
Copper and selenium are not heavy metals but may be present in eggs due to their authorised uses as PPPs and feed additives. These metals have a lower toxicity than heavy metals but may present a human health concern dependent on the level of presence. Further assessment would be required to determine whether these metals if detected in eggs at a specific level, would be a risk to consumers.
4.5. Microbiological hazards
The microbiological hazards shortlisted for characterisation were Salmonella spp, Campylobacter spp, and Listeria monocytogenes. Conclusions on the concerns regarding the presence of microbiological hazards are presented in section 4.5.4.
4.5.1. Salmonella
The genus Salmonella contains two species, S. enterica and S. bongori. Of those, Salmonella enterica serovars Enteritidis (SENT) and Typhimurium (STM) (non-typhoidal serovars) are the main types that are transmitted via food consumption (WHO, 2018b). References to Salmonella in this section encompass both non-typhoidal serovars -SENT and STM – unless a serovar is specified.
Salmonella spp growth conditions can be found in Table 5. The pH and aW in the internal parts of the egg as described in section 2.2.3, are within the range that permits the growth of Salmonella spp. However, for such growth to occur other conditions such as temperature, oxygen and salt level also need to be favourable.
Salmonella spp are readily destroyed by pasteurisation temperatures. This is affected by the food matrix, however, for example in low aw foods, such as peanut butter, the survival of Salmonella spp at 70 °C is increased (Beuchat, Komitopoulou, et al., 2013). Dried egg products fall within this low aW food category. The minimum water activity that permits growth of Salmonella spp is 0.93 however cells are able to survive in dried foods for extended periods of time (Beuchat, Komitopoulou, et al., 2013).
4.5.1.1. Hazard route
In 2022 in the UK, SENT was most frequently isolated from chickens compared to other animals, whilst STM was most frequently isolated from pigs (APHA, 2023). A list of the most commonly isolated serovars and phage types can be found in a report by the Animal and Plant Health Agency (APHA) (APHA, 2023). These patterns are likely to be different in different parts of the world, and they can affect the types of mitigations required, as transmission routes may differ. For example STM is the most prevalent serovar in hens in Australia (Moffatt & Musto, 2013; WHO, 2018b), whereas SENT is the most commonly found serovar in hen environments in other parts of the world (Whiley & Ross, 2015). However, STM has been associated with outbreaks in duck eggs in the EU (EFSA, 2014c).
There are two mechanisms whereby eggs can become internally contaminated with Salmonella; primary and secondary contamination.
Primary contamination (vertical transmission) occurs when Salmonella contaminates an egg during its formation. SENT is the serotype most associated with egg contamination by this means, due to its genetic makeup that enhances its ability to colonise the ovary and oviduct of laying hens (EFSA, 2014c). Although, STM has also shown ability to colonise the internal organs of the hen or invade into the egg albumen and yolk (EFSA, 2014c).
Secondary contamination (horizontal transmission) occurs when Salmonella contaminates the surface of an egg after its formation. This can be due to contact with contaminated surfaces, or contaminated feed and water, and insufficient cleaning of processing equipment and food contact surfaces (DEFRA, 2007). This contamination is facilitated by cracks on the eggshell. Such cracks can be caused by practices such as poor egg collection systems, and rapid cooling of eggs that may be practiced to slow down growth of any Salmonella which may be present (DEFRA, 2007; EFSA, 2014c).
Testing of UK shell eggs for Salmonella prevalence has not been conducted in recent years, however a 2003 FSA study found the prevalence of Salmonella (all serotypes) to be 0.34% (as reported in (FSA, 2023b).
Once in the egg, Salmonella needs to reach the yolk in order to grow and multiply. Migration of Salmonella from the albumen into the yolk depends on contamination dose, temperature, and age of the eggs (EFSA, 2014c). The growth rate of Salmonella within the egg environment depends on its location and is highly temperature dependent. SENT is unable to grow below 7°C, in egg albumen it cannot grow below 8°C and in egg yolk weak growth was observed at 10°C. Between 20 to 30°C, the growth of Salmonella is possible in all egg components. Overall, growth of Salmonella is faster in yolk than in albumen (EFSA, 2014c).
Sources of Salmonella introduction on farms are varied, evidence suggests that contaminated feed and water, pests such as rodents and insects, and introduction of infected hens, can cause flock contamination (FSANZ, 2013).
Salmonella naturally infects and colonises chickens, with conflicting studies failing to identify the key risk factors (likely due to the number of variables). However suggested risk factors include flock size, with larger flocks more likely to be infected with Salmonella; farm size, with larger farms less likely to use deep clean production systems; and on-floor housing systems. These systems increase the likelihood of Salmonella prevalence (ACMSF, 2016).
The risk factors associated with each of the egg production steps as identified in the British Red Lion Eggs Scheme are detailed in the FSA risk profile of UK produced hen shell eggs (BEIC, 2013; FSA, 2023b) and summarised in Table 6.
4.5.1.2. Hazard characterisation
With respect to observed cases of illness, the two most important serotypes, SENT and STM are transmitted from animals to humans across the world (Majowicz, Musto, et al., 2010). Globally, there are an estimated 93.8 million cases of gastroenteritis due to non-typhoidal salmonella infection each year, resulting in approximately 155,000 deaths, of which approximately 85% are estimated to be foodborne (Majowicz, Musto, et al., 2010). S. Enteritidis is considered the most important transovarian serotype concerning eggs and egg products, however, other serotypes which are not capable of transovarian transmission, such as S. Typhimurium, S. Heidelberg, and S. Infantis can also infect laying hens and contaminate eggs, to a lesser extent (EFSA, 2014c).
Infections by both STM and SENT are characterised by a self-limited gastroenteritis which is accompanied by symptoms including non-bloody diarrhoea, vomiting, nausea, headache and abdominal cramps (Zhang, Kingsley, et al., 2003). The onset of disease symptoms occurs after 6-72 hours (usually 12-36 hours) after ingestion of Salmonella, and illness lasts 2-7 days. The severity of Salmonella infections in humans varies depending on the serotype involved and the health status and age of the patient (Scallan, Hoekstra, et al., 2011).
Salmonellosis is generally an acute disease, however chronic illnesses can occur post-infection. It is estimated that 0.3% to 6.2% of cases can develop irritable bowel syndrome (IBS), referred to as post infectious IBS and around 5.8% can develop reactive arthritis (Keithlin, Sargeant, et al., 2015). According to UK Health Security Agency (UKHSA) data, between 2015 and 2019, a total of 954 outbreak related cases were confirmed cases of salmonellosis, associated with the consumption of eggs and/or egg products (FSA, 2023b). Non-typhoidal salmonellosis is classified as a serious hazard by the International Committee on Microbiological Standards for Foods (ICMSF); incapacitating but not life-threatening; sequelae infrequent; moderate duration (ICMSF, 2018).
Salmonella spp (primarily SENT) was the most frequently reported hazard in the hazard identification searches, and the primary cause of outbreaks associated with the consumption of eggs. Eggs and egg products were the primary vehicle of Salmonellosis outbreaks (26%) in the UK in the years 2015 – 2020 (DEFRA, 2023). Of those, 68% were associated with S. Enteritidis (FSA, 2023b).Eggs and egg products were the primary vehicle of Salmonella outbreaks (44%) in the EU in 2021 (EFSA, 2022a). Between 2017 to 2021, the prevalence of SENT in laying flocks in the UK, determined via national control programme (NCP) testing, was 0.19% (FSA, 2023b). In the EU, NCP results in 2022 determined an overall prevalence of SENT to be 0.15% in laying flocks, and an overall prevalence of STM to be 0.10% in laying flocks (EFSA, 2023f).
4.5.2. Campylobacter
4.5.2.1. Hazard route
Campylobacter spp growth conditions can be found in Table 7. The pH and aw in the internal parts of the egg as described in section 2.2.3, are within the range that permits the growth of Campylobacter. However, for such growth to occur, other conditions such as temperature, oxygen and salt level also need to be favourable.
Of the Campylobacter species, C. coli and C. jejuni account for over 95% of human infections (Park, 2002). They are prevalent in many domesticated animals, but their main reservoir is poultry (Sahin et al., 2002), as the body temperature of these animals (42oC) and the amino acids in their gut create a favourable growth environment (ACMSF, 2019). A study in Italy showed that the prevalence of Campylobacter in laying hens to be up to 94.6% (in aviaries), with caged birds showing the lowest prevalence (86.7%) (Casalino, Bozzo, et al., 2022). The lower levels seen in caged birds was believed to be due to the lower exposure to faeces. Lower prevalence has also been reported when the cloaca and eggshells were sampled instead of faeces (Gharbi, Bejaoui, et al., 2022; Guyard-Nicodème, Anis, et al., 2023).
The BfR have reported that chicken eggs may transmit Campylobacter to humans if the eggs are visibly contaminated with chicken excrement, due to excrement adhering to the shell of chicken eggs during production and packaging (BfR, 2018a). Eggs visibly contaminated with faeces or any excrement are not allowed to be sold at retail, however this contamination may not be visible (Dorn-In, Daldrup, et al., 2024).
Campylobacter transmission to the birds via drinking water, feed, old litter, pets, other animals, insects, equipment and farm workers have been investigated and is believed to be the main route of exposure (ACMSF, 2005, 2019; Hakeem, Fathima, et al., 2022). However, some studies have shown that Campylobacter can be transmitted directly to the chicks or to the egg before reaching the chicks (pseudo-vertical transmission), although this route is still believed to have a minor effect (ACMSF, 2005, 2019).
Occurrence of Campylobacter on eggshells has been reported (Adesiyun, Offiah, et al., 2005; BfR, 2023; Messelhäusser, Thärigen, et al., 2011; Sabzmeydani et al., 2020). However, no reports were identified on the organism being isolated from the albumen or yolk unless the eggs were cracked, artificially inoculated or put into contact with contaminated surfaces (Fonseca, Beletti, et al., 2014). Although the survival of the organism in artificially inoculated egg parts has been confirmed in some studies (Shane et al., 1986), the result could not be replicated in others (Paula, Fonseca, et al., 2009).
In a metanalysis on source attribution for campylobacteriosis, Fravalo et al. identified eggs (particularly raw eggs and mayonnaise) as significant risk factors for campylobacteriosis. The study proposed that if Campylobacter is present on the eggshell, it could contaminate the egg during cracking, posing a risk of infection when the egg is consumed undercooked (Fravalo, Kooh, et al., 2021). Dorn-In et al. artificially contaminated eggs with C. jejuni. They found cross-contamination in 68% of egg whites and 14% of egg yolk after separation, and consequently recommend avoiding the manual separation of egg white and yolk using the eggshell (Dorn-In, Daldrup, et al., 2024).
Risk factors that contribute to Campylobacter colonisation of poultry, include movement of birds on farms during depopulation, movement between broiler houses and transfer by staff were the three top contributing factors to Campylobacter occurrence (Adkin, Hartnett, et al., 2006). The size of the flock, the number of bird houses, the use of manure, and water coming from centralised water systems (Guerin, Martin, et al., 2007). While these studies looked at broiler farm practices the risk factors identified are likely to affect layer flocks when practices are similar. Climatic conditions may be an important risk factor, with hot climates favouring higher prevalence of Campylobacter and higher microbial load in the birds while colder climates are likely to have the opposite effect (ACMSF, 2005; Sibanda, McKenna, et al., 2018). However, this was not the case in Iceland (Guerin, Martin, et al., 2007).
4.5.2.2. Hazard characterisation
Campylobacter (mainly C. jejuni and C. coli) causes campylobacteriosis in humans, a leading cause of bacterial gastrointestinal illness in many countries (ICMSF, 2018). The most common clinical symptoms of Campylobacter infections include diarrhoea (frequently bloody), abdominal pain, fever, headache, nausea, and/or vomiting, with symptoms typically lasting 3 to 6 days (WHO, 2020a). In rare cases, campylobacteriosis can be the cause of long-term conditions such as the Guillain-Barre syndrome, rheumatological conditions (ACMSF, 2005).
In terms of severity, the ICMSF categorise Campylobacter jejuni as “Moderate, not usually life-threatening; no sequelae; normally short duration; symptoms are self-limiting; can be severe discomfort” (ICMSF, 2018). Moderate is the lowest category used by the committee.
Campylobacteriosis is the most commonly reported foodborne gastrointestinal infection in humans in the EU and has been so since 2007 (EFSA, 2022f). In 2021, 127,840 confirmed cases of human campylobacteriosis were reported in the EU (EFSA, 2022f). The results reported in 2021 by 16 member states (MS) for non-ready to eat food show that ‘meat and meat products’ (including poultry) was the most contaminated food category (EFSA, 2022f).
Outbreaks of campylobacteriosis are rare, with most cases being sporadic and commonly attributed to undercooked poultry or cross-contamination from raw poultry (ICMSF, 2018). Fresh poultry meat is the most frequent source of human Campylobacter infections (BfR, 2018a; ICMSF, 2018). A recall of chicken eggs due to presence of C. coli was reported by BfR in 2019 (FERA, 2024; FoodAkai, 2024). Although Campylobacter outbreaks have been attributed to eggs, a direct link has not been established so far (ACMSF, 2016).
4.5.3. Listeria monocytogenes
4.5.3.1. Hazard route
Listeria monocytogenes is a species of facultatively anaerobic non-spore forming bacteria. Its growth conditions can be found in Table 8. The pH and aw in the internal parts of the egg, as described in section 2.2.3, are within the range that permits the growth of L. monocytogenes. However, for such growth to occur, other conditions such as temperature, oxygen and salt level also need to be favourable.
There are many reservoirs of L. monocytogenes, and unlike most other foodborne pathogens, they can live and grow in the natural environment without the need to grow within an animal host (Chasseignaux, Toquin, et al., 2001).
Poultry themselves can harbour L. monocytogenes and contaminate their environment (Rothrock, Davis, et al., 2017). This pathogen has been isolated from environmental samples from both broiler (Rothrock, Davis, et al., 2017) and layer farms (Ricke et al., 2023), including from samples such as from litter, dust, grass, feed, faeces and caecal content and cloacal swabs. The highest incidence was detected in faecal samples in both broiler and layer farms (Ricke et al., 2023).
Prevalence rates on eggshells has been found to be low (1.8 - 6%) (Ricke et al., 2023). In another study in Mexico, L.monocytogenes was isolated from 4.6% of eggshell samples. It has also been isolated from the eggshells of inoculated eggs and the population was found to decrease over storage for 6 weeks. The decrease was faster at 20oC compared to 5oC (Brackett & Beuchat, 1992).
The risk factors identified in broiler environments were related to hygiene and biosecurity (separation of clean and dirty areas, no pest control, staff visiting other broiler houses, presence of pets, type of feed and failure to remove faecal matter, etc) (Rothrock, Davis, et al., 2017). While layer rearing systems can differ significantly from broiler systems, such risk factors are likely to affect the prevalence of environmental pathogens such as L.monocytogenes in similar ways.
Environmental cross-contamination is a major issue with respect to L. monocytogenes, in particular when the egg is first cracked before downstream processing (Brackett & Beuchat, 1992). It can occur through direct contact with raw materials, personnel, aerosols and contaminated utensils, equipment, and other surfaces that may come into contact with food (FSANZ, 2013). Cross-contamination can occur at any step where the product is exposed to the environment, including processing, transportation, retail, catering and in the home. Therefore, L. monocytogenes is a pathogen primarily associated with ready to eat foods (FSANZ, 2013).
Because L. monocytogenes is a hazard which can be introduced into food products from the processing environment, the majority (83%) of alerts recorded in the FSA RLD (including RASFFs, UK, Canada and US FDA alerts) are related to egg products rather than shell eggs.
L. monocytogenes have been detected in raw liquid whole egg samples at a 17.4% prevalence before pasteurisation and 2.1% after (Rivoal, Quéguiner, et al., 2010). The contamination of the pasteurised products did not indicate that the pathogen survives pasteurisation, but rather post processing contamination. This is because the corresponding raw products were not found to contain the pathogen. A later study by the same authors showed no contamination in pasteurised eggs and an 8.5% of incidence in raw products (Rivoal, Fablet, et al., 2013).
In the context of egg products, risks of contamination post pasteurisation may come from the environment (for instance, due to improper cleaning and sanitation allowing L. monocytogenes to establish and form biofilms) or from employees inadvertently carrying L. monocytogenes on their person or clothing (USDA, 2020).
4.5.3.2. Hazard characterisation
Infection by L. monocytogenes is known as listeriosis. Various clinical manifestations are associated with L. monocytogenes infection, and these can be grouped in two categories: invasive and non-invasive listeriosis.
Non-invasive listeriosis typically occurs, is generally mild and manifests as diarrhoea, fever, headache and myalgia (WHO, 2018a). During a number of outbreaks, the majority of cases developed symptoms of gastroenteritis after a short period of incubation (Aureli, Fiorucci, et al., 2000). Non-invasive listeriosis has not been well studied as the clinical presentations do not typically warrant medical intervention and are therefore not reported (CDC, 2024). Non-invasive listeriosis is typically self-limiting and symptoms only last a few days (CDC, 2024).
In contrast, the symptoms of invasive listeriosis are severe, and include fever, myalgia (muscle pain), septicaemia, and meningitis with high mortality rates (WHO, 2018a). The incubation period is usually one to two weeks, but can vary from a few days to 90 (WHO, 2018a). According to one outbreak report in a tertiary care hospital, the incubation period was 3-4 days which was significantly shorter than other reports (Johnsen, Lingaas, et al., 2010).
Invasive listeriosis presents a particularly serious risk to pregnant women including unborn babies, people with weakened immune systems and elderly people, where it can cause very severe illness and death (De Luca, Donati, et al., 2015). The case fatality rate of invasive listeriosis is high, ranging from 20-30% (Mead, Slutsker, et al., 1999). Pregnant women infected with L. monocytogenes can experience miscarriage, stillbirth and premature birth, which while not typically fatal for the mother can be fatal for the fetus (Pezdirc, Hure, et al., 2012).
ICMSF classified listeriosis as serious hazard; incapacitating but not life-threatening; sequelae infrequent; moderate duration. No differentiation was made between invasive and non-invasive listeriosis (ICMSF, 2018).
Egg associated outbreaks of L. monocytogenes are not frequently reported, however, such an outbreak in the USA in 2017-2019 resulted in multiple human cases and 1 death. The implicated product was hard boiled eggs intended for food processors and restaurants (CDC, 2020).
4.5.4. Conclusion
Overall, microbiological hazards present a risk in EEPs, with SENT being by far the most frequently implicated in alerts and incidents. Enteric pathogens such as Campylobacter and Salmonella will naturally infect chickens if they are exposed, typically via contaminated feed, water, or from exposure to environmental sources, for instance wild animals or insects. Listeria monocytogenes can also contaminate from this enteric route, however it also has the ability to persist in the environment, and can establish a presence in a manufacturing environment, where it can contaminate egg products.
Human health effects of these pathogens vary depending on exposure and consumer group. If these pathogens are found to be present in eggs, there may be a concern for consumer health and further assessment would be required.
4.6. Pesticides
4.6.1. Hazard route
Pesticides in the form of PPPs are applied to plants to control pests, weeds and plant diseases. As poultry feed contains cereal grains, cereal by-products and plant protein sources among other ingredients (FAO, 2024), there is potential for ingestion of pesticides through the diet. When hens consume contaminated feed, the pesticides or their metabolites may accumulate and can be distributed to eggs which is evidenced by animal metabolism and feeding studies (JMPR, 2023).
In addition, pesticides can enter eggs through environmental exposure of laying poultry, such as through contaminated soil. Field studies showed that raising hens in contaminated areas might lead to transfer of pesticides from soil to the eggs (Piskorska-Pliszczynska et al., 2019). According to the EU pesticide report 2021, the detected presence of the (now non-approved) pesticide chlordecone in chicken eggs relates to its persistence in the soil. Contamination may occur in chickens in open-cage farms when feed is placed in contact with the soil in areas where this active substance was previously used (EFSA, 2023e).
4.6.2. Hazard characterisation
The toxicity of pesticide residues and the potential adverse effects that may result from unacceptable levels of exposure, depends on the toxicological profile of the substance, the dose-response relationship, and the level of exposure.
Pesticides are regulated in eggs by monitoring residue levels in relation to MRLs under assimilated Reg. (EC) 396/2005 (European Parliament, 2005; Gov UK, 2005). Where pesticides are used in accordance with good agricultural practice (GAP) this is unlikely to be of concern, as levels in poultry and eggs are expected to be below the MRL. Occurrence of a residue below the relevant MRL indicates that an unacceptable risk to consumers is unlikely. However, an exceedance of an MRL does not necessarily indicate a concern for consumer health, it is typically taken as a trigger for risk assessment or other enforcement action.
Appendix VIII summarises the pesticides detected in the literature and the highest levels reported. As the pesticide residues identified in the literature review were often not quantified and consistent reporting was not available, EU Pesticide Monitoring Reports, UK Pesticide Residues in Food (PRiF) Reports, and incidents and alerts data was used to identify potential pesticide residue MRL exceedances. Table 9 summarises the non-compliant pesticide residues that exceeded their MRLs in eggs and egg products in the EU and UK between 2019 and 2024.
Two of the pesticides identified in eggs also have biocidal and/or veterinary medicine uses, fipronil and chlorate (residue from chlorine-based disinfectants), and residues detected in eggs likely arose from these uses. Further discussion can be found in section 4.2.
4.6.3. Conclusion
Overall, a relatively small number of non-compliant cases due to pesticide residues have been detected in eggs.
The potential adverse effects that may result from exposure to residues of pesticides depends on the toxicological profile of the substance, the dose-response relationship and the level of exposure. The information available from EU and UK monitoring, alerts and incidents data, indicate that some pesticide residues may be present at levels above their respective MRLs. The presence of pesticide residues in eggs exceeding the MRL, or presence of a residue of a pesticide that is not authorised, may require further action in the form of risk assessment to determine the risk to consumer.
4.7. Veterinary medicines and feed additives
4.7.1. Hazard route
The deliberate addition of veterinary medicines and feed additives to animal feed is an important route for administering these substances to animals, in particular to animals intended for food production (Codex, 2019). Other application routes of veterinary drugs are also possible, such as via drinking water or injection (ACAF, 2007).
In GB antiprotozoal agents (coccidiostats and histomonostats) are permitted as feed additives but the use of antibiotics in feed additives is prohibited (FSA, 2024a). In some countries, in addition to treating infections, antibiotics are added to feed as growth promoters, although in the UK, it is illegal to use any antibiotic as a growth promoter (FSA, 2024b; WOAH, 2022).
The unintended carryover of veterinary drugs from medicated feed to un-medicated feed at feed manufacturing facilities can be a source of contamination (Codex, 2019). It is also possible for a non-target animal to be given feed formulated for a target animal species, intentionally or accidentally.
In one study, lasalocid content in eggs was analysed. Lasalocid is a coccidiostat, which at the time of the study was not authorised for use in laying birds. Approximately 66% of the eggs analysed contained lasalocid at levels correlating to the concentration in the feed. Carryover of lasalocid from manufacture of medicated broiler and turkey feeds to unmedicated layer feed in local feed mills was identified as the possible source of the residues in eggs (Kennedy, Blanchflower, et al., 1996). Another study also concluded that unintentional and unavoidable carry-over of nicarbazin could affect laying hens, most-likely as the feed for chickens (for fattening) and laying hens is often prepared at the same mill (Codex, 2023).
A study has shown that ingestion of veterinary drugs such as coccidiostats by laying hens can lead to residues in eggs (Goetting et al., 2011). During egg development in poultry, precursors to yolk lipoproteins are produced in the liver and transported to the yolk follicles in the ovary. The veterinary drugs that deposit in the egg yolk rapidly accumulate during this time and can be present in successive eggs for 10 or more days following treatment. Following yolk maturation, the albumen is laid down and can also serve as a residue accumulation site. A variety of drugs left detectable residues in eggs laid days to weeks after the cessation of treatment (Goetting et al., 2011).
4.7.2. Hazard characterisation
The toxicity of veterinary medicine or feed additive residues and the potential adverse effects that may result from unacceptable levels of exposure depends on the toxicological profile of the substance, the dose-response relationship, and the level of exposure. The potential adverse effects of veterinary medicine residues in food are broad, but include allergic reactions, nephrotoxicity, hepatoxicity, reproductive disorders, bone marrow toxicity and carcinogenicity (Bacanli & Basaran, 2019; Baynes, Dedonder, et al., 2016; Hosain et al., 2021).
Some of the antibiotics used in poultry can also be prescribed for human use, for example ciprofloxacin and ofloxacin which are used to treat serious infections in humans (MHRA, 2024). Overuse and misuse of antibiotics contributes to antimicrobial resistance, which leads to antibiotics becoming ineffective and infections becoming more difficult to treat (WHO, 2023a).
Veterinary medicine residues in food and feed are controlled according to MRLs in GB (VMD, 2024b) and NI (European Commission, 2009c). EU monitoring of VMP residues in live animals and animal products showed coccidiostats were reported to be non-compliant in 0.11% of the samples analysed, with highest occurrence in rabbit meat (0.87%) followed by eggs (0.42%) (EFSA, 2023c).
Appendix IX summarises the veterinary medicines and feed additives detected in EEPs, and the highest levels reported. Table 19 and Table 20 show the non-compliant cases of coccidiostats and veterinary medicine residues in eggs identified from the UK annual surveillance results on residues of veterinary medicines in food between 2019 and 2024 (up to 30 June), EU annual reports on VMP residues in food between 2019 and 2021, and other incident information sources such as RASFF (EFSA, 2024b), as well as residues identified in the literature.
Chloramphenicol and nitrofuran were identified in eggs via the literature review and alerts (EFSA, 2014a). Both are prohibited substances in food-producing animals in the EU and UK (European Commission, 2009c; VMD, 2024b). EFSA has concluded that there is no safe level of residues of chloramphenicol and nitrofuran or their metabolites in food that represents an acceptable risk to consumers (EFSA, 2014a, 2015b). Therefore, although chloramphenicol and nitrofuran metabolite AOZ have been found in eggs, they are prohibited substances and therefore are excluded for further assessment.
4.7.3. Conclusion
Residues of veterinary medicines and feed additives may be present in EEPs. Many residues detected in eggs were coccidiostats and histomonostats which are predominately regulated as feed additives in the UK, although some are also regulated as veterinary medicines.
The potential adverse effects that may result from exposure to VMPs and feed additives depends on the toxicological profile of the substance, the dose-response relationship and the level of exposure. The information available from EU and UK monitoring, alerts and incidents data indicate that some residues may be present at levels above respective MRLs. Additionally, residues of VMPs or feed additives which are not authorised, or which are specifically prohibited such as chloramphenicol, may be present and these may be a concern for consumer health. The presence of a residue of VMP or feed additive in eggs which is not authorised or exceeds the MRL, may require further action in the form of risk assessment to determine the risk to consumer.
5. Hazard Prevention, Mitigation and Controls
5.1. Hazard Mitigation
Interventions designed to eliminate or reduce the amount of any given hazard present in EEPs can be applied at a number of stages throughout the production process; from the farm where the poultry feed grows, to the retail market for the final product. Such activities aim to either prevent the hazard from entering the EEPs or mitigating the hazard before the final product reaches the consumers. Mitigations that the consumers can take at home, such as cooking, are not discussed in this section, as they would be the same for domestic and imported EEPs and they are not in scope.
In the rest of this section, mitigations are discussed in the context of how hazards may enter the EEPs.
5.2. Hazards introduced during egg formation
Hazards that may enter the egg before it is laid include pathogenic microorganisms such as Salmonella Enteritidis and a number of chemical hazards. These two groups of hazards (microbial & chemical) are discussed separately below.
5.2.1. Chemical hazards
Chemical hazards that may enter the egg during its formation are taken up by the birds via feed, water or contamination in the farm environment (e.g. in soil or bedding). These hazards include mycotoxins, PAs, environmental contaminants, metals, pesticides and veterinary medicines.
Mitigation measures for chemicals in eggs and egg products are primarily related to the environment of the poultry farm and the poultry feed and water, as there are currently no identified mitigation measures in the egg, other than monitoring. Quality control plans can be used to address this, in the UK this includes setting sampling and testing frequencies for all feed ingredients and feeding stuffs (BEIC, 2013).
5.2.1.1. Animal feed & water
Poultry diets are formulated from a mixture of ingredients, including cereal grains, cereal by-products, fats, plant protein sources, and other nutrients (FAO, 2024). Agricultural contaminants, pesticides, veterinary medicines and feed additives primarily enter the eggs via this route.
The Codex “Code of Hygiene Practice on Good Animal Feeding” provides recommendations on prevention of contaminants in eggs, as improper procurement, manufacturing and handling of animal feed may result in the introduction of hazards into the eggs and egg products (Codex, 2004). This is expanded on in the FAO “Good practices for the feed sector guidance” which makes recommendations around feed ingredients, traceability, inspection and control and GMP as well as feed production (FAO, 2020).
In the Codex “General Standard For Contaminants And Toxins In Food And Feed”, maximum levels of various chemical contaminants are specified in feed, including aflatoxins, and ochratoxin A, melamine and several metals (Codex, 1995). This standard specifies that contaminant levels should be as low as possible, achieved by following GAP and good manufacturing practice (GMP), with specific actions recommended. For example preventing contamination at source (e.g. by reducing environmental pollution) and applying measures to prevent marketing of contaminated feed for consumption.
5.2.1.1.1. Mycotoxins
The Codex Alimentarius recommend a number of measures for the reduction of mycotoxin contamination in cereals, including appropriate use of crop rotation, use of appropriate pesticides and ensuring grain is dried appropriately (Codex, 2003).
The FSA has produced two guidance documents regarding mycotoxins, specifically fusarium and ochratoxin A in cereals. Specific mitigations recommended are avoiding maize in crop rotation, use of fungicides and appropriate drying and grain storage (FSA, 2007a, 2007b).
The FAO have produced “On-Farm Mycotoxin Control in Food and Feed Grain”, which provides guidance on; signs of mycotoxins and mould growth, factors that affect fungal growth, symptoms of mycotoxin poisoning in poultry, and how to prevent mould damage before, during and after harvest (FAO, 2007).
Bentonite (a type of clay) can be added to feed to bind with mycotoxins in the digestive tract, thereby reducing mycotoxin contamination of animal products (EFSA, 2011b). The maximum content of bentonite in feed is controlled by UK legislation (European Commission, 2013).
In GB and NI maximum permissible limits are set for certain aflatoxins in feed under regulations GB 2015/255 and 2002/32/EC respectively, and guidance values are set for OTA. The maximum permissible level of AFB1 in feed materials (based on a moisture content of 12%) is 0.02 mg/kg (European Parliament, 2002; Gov UK, 2002, 2015c). The upper guidance value for ochratoxin A in cereals and cereal product feed materials (based on a moisture content of 12%) is 0.25 mg/kg, and for compound feed for poultry 0.1 mg/kg (European Commission, 2006).
Mitigation measures for toxins in EEPs are related to the poultry feed, as there are currently no identified mitigation measures in the egg, other than monitoring. Quality control plans can be used to address this, in the UK this includes setting sampling and testing frequencies for all feed ingredients and feeding stuffs (BEIC, 2013).
5.2.1.1.2. PAs
Codex Alimentarius has set out a code of practice to reduce PA contamination (Codex, 2014). This involves weed management (removal/reduction) practices of PA containing plants, mechanical methods such as pulling or ploughing, chemical methods such as the use of herbicides and using biological agents.
5.2.1.1.3. Pesticides
In the UK and EU, pesticides must be authorised before being placed on the market. Authorised uses must be supported with a satisfactory risk assessment which includes a consideration of potential levels in poultry and eggs and subsequent consumer exposure. Pesticides are required to be appropriately labelled with instructions for use and must be used in accordance with GAP (HSE, 2024f). In GB, the Health and Safety Executive (HSE) is responsible for MRLs for pesticides. An MRL is the maximum concentration of a pesticide residue in food of plant or animal origin that is legally tolerated when a pesticide is applied following good agricultural practice (assimilated Reg. (EC) 396/2005) (European Parliament, 2005; Gov UK, 2005). Where pesticides are used according to the conditions of authorisation, residues exceeding the MRL in eggs should not occur.
An import tolerance is an MRL set on imported food or feed to meet the needs of international trade. Where a commodity is treated with a pesticide at a higher rate internationally than in GB, and the commodity is imported into GB, an assessment of the risk and expected levels in the commodity must be made, with an import tolerance set if required (HSE, 2024e).
Globally, the majority of countries set their own national MRLs, but in addition Codex Alimentarius sets Codex maximum residue levels (CXLs) for pesticides which are internationally agreed food standards (Codex, 2024a).
The FAO “International Code of Conduct on Pesticide Management (the Code of Conduct)” serves as a voluntary framework for all public and private entities engaged in, or associated with, production, regulation and management of pesticides (FAO, 2014).
There are pesticide monitoring programmes globally, including in GB and EU, which monitor pesticide residues in a range of crops (DEFRA, 2022b; European Commission). The results of the monitoring programmes can be used to target further action.
For eggs specifically, Codex states that appropriate feed must be used so as not to introduce chemical hazards such as pesticide residues into eggs (Codex, 1976). In the code of practice for animal feeding, it is specified that feed ingredients must be within statutory levels for pesticides (Codex, 2004).
The EU Drinking Water Directive sets maximum allowable concentrations of 0.1 µg/L for any pesticide and 0.5 µg/L for total pesticides in drinking water, irrespective of toxicity (European Parliament, 2020). WHO guidelines also provide some recommendations for mitigations including system plans and process control measures (WHO, 2022b).
5.2.1.1.4. Veterinary medicines
In GB, the Veterinary Medicines Directorate (VMD) is responsible for MRLs for veterinary medicines. The MRL is the maximum allowed concentration of a residue in a food product obtained from an animal that has received veterinary medicine or that has been exposed to a biocidal product for use in animal husbandry. Where the veterinary medicine is applied to the animal at up to the maximum allowed dose rate, residues exceeding the MRL should not occur.
In GB, VMD carries out an annual surveillance plan to analyse samples from food producing animals for residues of authorised veterinary medicines, prohibited substances and various contaminants (VMD, 2024d). In addition, farm inspections on the use of veterinary medicines are conducted (VMD, 2023b). In the EU, the EMA has a similar role, and also undertakes surveillance (EMA, 2024). Codex has produced guidelines for implementing a regulatory assurance programme for use of veterinary drugs in livestock (Codex, 2009).
WHO has launched new guidelines on the use of medically important antimicrobials in food-producing animals, recommending that farmers and the food industry stop using antibiotics routinely to promote growth and prevent disease in healthy animals. These guidelines aim to help preserve the effectiveness of antibiotics that are important for human medicine by reducing their use in animals (WHO, 2017b).
As highlighted in section 4.7.1, cross-contamination between feed for target species containing between coccidiostats and histomonostats and feed for non-target species can occur during preparation at the same mill.
Codex guidance states that feed additives and veterinary drugs used in medicated feed, should be assessed for safety and used under stated conditions set by competent authorities (Codex, 2005). It advises clearly distinguishing between feed additives and veterinary drugs used in medicated feed, to avoid misuse. This can be considered critically important to prevent carry-over and cross-contamination of medicated feed with non-medicated feed for non-target species (Codex, 2005).
It is essential that levels of undesirable substances are sufficiently low in feed and feed ingredients, that their concentration in food for human consumption, including eggs is consistently below the level of concern. Overall to achieve this, below points needs to be taken into account:
-
Following Codex guidelines, GMP (Codex, 2005)
-
Regular monitoring and identifying coccidiostats and histomonostats in feed (European Parliament, 2002) and egg above the MRL (VMD; European Commission, 2009b)
-
Usage according to recommended withdrawal times (European Commission, 2009a)
-
Usage for species that it is only authorised for (VMD; European Commission, 2009c, 2009b, 2012; Gov UK, 2009a, 2012)
-
Measures to prevent cross-contamination during manufacturing, storage, transport and usage (e.g., carry-overs in mills) (Codex, 1995, 2005, 2023).
5.2.1.2. Environmental
Contamination of eggs with certain chemicals can occur through the environment of the poultry farm. Environmental contaminants include dioxins, dioxin-like substances, PCBs, PFAS, and metals. For these chemicals, environmental exposure is the primary route of contamination, which in turn may contaminate the poultry feed and water (see section 5.2.1.1).
5.2.1.2.1. Environmental contaminants
Codex guidance on source directed measures to reduce chemical contamination of food, notes that pollution can result in the contamination of crops grown for feed and drinking water, and that appropriate measures for national authorities may be to (Codex, 2001):
-
Control emissions of pollutants from industry
-
Control emissions from energy generation and means of transportation
-
Control the disposal of solid and liquid domestic and industrial waste
-
Control the production, sale, use and disposal of certain toxic, environmentally persistent substances
-
Ensure that before new chemicals are introduced, they have undergone appropriate testing to show their acceptability
-
Replace toxic environmentally persistent substances with alternatives
-
Codex also recommend that the surroundings and environment of the poultry farm should be considered for possible sources of contamination including, previous uses of the land, presence of chemical contaminants and polluted surface water (Codex, 1976).To reduce the contamination of dioxins and PCBs in eggs, control measures at the feed level should be considered. Such measures may include (Codex, 2018):
-
Identification of possibly contaminated areas in the feed supply ecosystem
-
Identification of the origin of frequently contaminated feed or feed ingredients
-
Monitoring the compliance of feed and feed ingredients with nationally established guideline levels or maximum levels, if available
The Stockholm Convention is a global treaty to protect human health and the environment from POPs. Of the POPs included, PFOS (a PFAS) and PCNs are targeted for elimination and the unintentional release of dioxins and PCBs must be minimised (Stockholm Convention, 2019).
EFSA determined PFAS transfer from feed to animal derived food, including eggs (EFSA, 2020e). As such, the European Commission has recommended further investigation in cases where MLs are exceeded in foodstuffs. This aims to determine the possible root cause of contamination, and to control feed, animal drinking water and the soil on which animals live, as well as undertake monitoring in food (European Commission). Measures can be taken to remove PFAS from drinking water (EEA, 2024).
Limited information is available with respect to the control or mitigations of PCNs or melamine in food or feed. EFSA reviewed the potential human health risks related to PCNs in food and feed, and made a recommendation that the monitoring of levels should be undertaken, particularly where animals are raised on PCN contaminated soil or near other sources (EFSA, 2024f).
Maximum legal levels, set in regulation at a national level, as outlined in section 4.3, are another way that the levels of contaminants in food products can be controlled.
Maximum levels for metals in drinking water in the UK are specified in The Water Supply (Water Quality) Regulations 2016 (Gov UK, 2016b). WHO provide information on the standards of water safety and recommendations for mitigations, including system plans and process control measures (WHO, 2022b). With regard to drinking water specifically for livestock and poultry, FAO guidance is available which provides guideline upper limits for toxic substances including metals in livestock drinking water (FAO, 1985).
5.2.2. Microbiological hazards
Most microbiological hazards that can be found in eggs are likely to come into contact with the egg after it is laid. However, Salmonella, and especially Salmonella Enteritidis can contaminate the egg during formation within the animal’s reproductive system. Campylobacter spp are believed to contaminate the egg surface when present in the egg environment. Contamination during the formation of the egg cannot be ruled out, albeit its effect is believed to be minor.
To mitigate hazards that colonise the birds, eradication and vaccination programmes, including for breeder flocks, are likely to be the most effective mitigation measures (OIE, 2022). All other mitigations discussed in section 5.3.2 can also support control efforts.
5.3. Hazards introduced after laying
5.3.1. Chemical
The main contamination route for chemicals in eggs after laying is during processing, where biocides may be used as disinfectants or insecticides.
5.3.1.1. Biocides
The Codex “Code of Hygiene Practice on Good Animal Feeding” states that cleaning programmes should minimise residues of detergents and disinfectants, and that machinery must be dried following wet cleaning (Codex, 2004).
Codex states that any pest control measures (which would include the use of biocides in Group 3: Pest control, e.g. PT18 insecticides and acaricides) should not result in unacceptable levels of pesticide residues in or on eggs and that properly designed pest control methods should be used (Codex, 1976).
For biocides used in animal husbandry which may leave residues in foods of animal origin, veterinary medicine MRLs are applicable, as set out in assimilated Reg. (EC) 37/2010, (European Commission, 2009c; VMD, 2024b). Where a biocide is currently or has previously been authorised as a pesticide in GB or EU (for example, fipronil and chlorate), then pesticide MRLs are applicable (assimilated Reg. (EC) 396/2005) (European Parliament, 2005; Gov UK, 2005).
5.3.2. Microbiological
From the hazards characterised herein, Salmonella Typhimurium’s primary route into the egg is via contact with contaminated surfaces, Listeria monocytogenes are primarily linked to cross-contamination in manufacturing settings, and Campylobacter spp, tends to colonise the egg shells when they come into contact with surfaces where the pathogen resides, primarily in faeces.
Controls for microbiological hazards in eggs are predominantly concerned with the control of Salmonella. These controls are discussed in detail below, but they can equally be applied to control other microbiological hazards, such as Campylobacter and Listeria monocytogenes.
In the UK, assimilated Regulation (EC) 2073/2005 (European Commission, 2005) sets requirements for sampling plans, analytical methods and permitted levels of microbiological hazards in foodstuffs for Salmonella and L. monocytogenes that can be found in foodstuffs. The requirements for EEPs can be found in Table 10.
In contrast to chemical hazards, microbiological hazards can be mitigated in industrial settings via heat treatment such as pasteurisation (USDA, 2020). Most egg products are pasteurised to destroy bacteria, however shell eggs are not always pasteurised. Shell eggs may be cooked, but they can also be eaten in circumstances where they are not cooked or not thoroughly cooked. Pasteurisation requirements for liquid whole eggs can vary by country. European heat treatments typically involve temperatures of 65 - 68 °C for 5 - 6 minutes for whole eggs and egg yolks. Egg whites undergo milder treatments (55 – 57 °C for 2 – 5 minutes) due to their higher heat sensitivity (EFSA, 2014c).
Controls for Salmonella in egg products include heat treatments to provide at least a 5-log reduction of viable cells, as well as cooling and freezing to prevent the growth of cells (USDA, 2020). Post lethality handling and sanitation is also important, which should be considered in HACCP systems, including microbiological sampling and testing. Verification of adequate pasteurisation should also be conducted, via testing for presence of Salmonella spp. (USDA, 2020).
The Food Safety and Inspection Service (FSIS) of the USDA guidance recommends that the presence of Salmonella in finished products can indicate pasteurisation failure. which may mean other pathogens such as Listeria may also be present (USDA, 2020).
L. monocytogenes is an environmental organism, with a strong adherence ability and a biofilm former., This is likely to affect its resistance to eradication attempts and should be considered when cleaning programmes are designed at an industrial setting (Gonzales-Fandos et al., 2021).
Campylobacter are susceptible to drying, freezing, heating, disinfectants and acidic conditions (pH<4.7) (FSS, 2019). However, resistance to disinfectants increases by several days when the cells form biofilm structures (ACMSF, 2019). Freezing reduces the microbial load significantly and chilling at refrigeration temperatures also results in slow reduction in bacterial numbers (ACMSF, 2019).
5.3.2.1. Salmonella
A control programme for Salmonella Enteritidis and Salmonella Tymphimurium in breeding flocks of domestic fowl has been in operation in the UK since 1989 (DEFRA, 2008). Control advice is provided, including on-farm visits, by experts in Salmonella control when appropriate (DEFRA, 2008).
Salmonella can be mitigated at the farm via various controls, including use of live attenuated vaccines of S. Enteritidis and other Salmonella serotypes. Evidence suggests that vaccines, whilst not completely protective, are effective in reducing the overall rate of Salmonella infection; faecal shedding; ovarian transmission, and the within-flock prevalence in hens (ACMSF, 2016).
Additional mitigations at the farm include preventing access of domestic, wild and feral animals to places where hens can access, as these animals can naturally carry Salmonella. Feedstuffs and water supply should be compliant with the respective country’s code of practice, as they can act as fomites to transmit Salmonella to hens. In addition, the environment should be kept clean, and surfaces disinfected; formaldehyde based disinfectants are most effective if organic matter is present (DEFRA, 2007).
The FSA matrix, which is used to assess industry schemes for effectiveness on Salmonella controls is discussed in section 2.2.4. The FSA matrix requires a number of controls for such schemes to be approved, including: biosecurity measures, pest control, cleaning and disinfection programmes on the farm, vaccination programmes for both breeding and laying flocks, limited use of antimicrobials to treat animal diseases, sampling and testing regimes throughout the production chain (from feed to the final product), personnel training, recording of all activities and controls, documented temperature controlled environment, independent auditing of documentation and clear processes when Salmonella is detected.
The Codex “Code of Hygienic Practice for Eggs and Egg Products”(Codex, 1976) includes similar key mitigation measures:
-
Environmental hygiene – ensuring the hazards in the production environment are minimised through measures such as waste management and pest control
-
Flock management and animal health – ensuring the flock is in good health via means such as vaccination (where vaccines exist, such as for Salmonella), flock checking and veterinary treatment when required
-
Hygienic production and handling – ensuring the establishment minimises exposure to hazards including via cleaning and disinfection, maintaining clean water, feed management and pest control
-
Personnel considerations – including ensuring appropriate training, personal hygiene and good health
-
Record keeping – ensuring traceability and accountability to verify the effectiveness of control systems.
Controls may differ depending on whether the type of prevalent Salmonella can be transmitted vertically or horizontally. For example, vertical transmission can be controlled with vaccination while for environmental transmission the source needs to be identified, and biosecurity measures may be more effective. Additionally, the ability of the pathogens to form biofilms should be considered when designing cleaning and disinfection regimes.
5.4. Hazard Controls
5.4.1. Import Conditions
EEPs can only be imported into the UK from countries that have market access approval and have an approved residue monitoring plan in accordance with EU Decision 2011/163 (GB) or Reg. (EC) 2022/2293 (NI) (European Commission, 2011, 2022b). Imported products must also be accompanied by appropriate health certificates based on the assimilated Reg. (EC) 2019/628 (European Commision, 2020).
5.4.2. Regulations applicable to the UK
Regulations relating to EEPs mainly concern general criteria such as: rules for public health requirements (including hygiene, microbiological criteria, contaminants regulations, additives regulations, use of antimicrobials). The regulations are mostly not specific to eggs but applicable to products of animal origin (POAO) more broadly.
There are also regulations and legislation specific to the majority of the hazards identified in this profile. Regulations in this section have been split into areas to which they relate e.g. hazard specific such as microbiological criteria or commodity (EEP) specific. Regulations noted in this section are in relation to both GB and NI, as EU legislation remains applicable to NI. Where Regulations fall under assimilated legislation, these may be the same. Where Regulations are relevant to NI only, this is noted in the first column of the table. Any Commission regulations noted in the table which are not noted as specific for the UK, GB or NI, will be applicable to all. Regulations with only UK, GB, Welsh or Scottish references are relevant only to these areas. Any Regulations referring to EU Exit are relevant to GB only.
Regulations listed and summarised in Table 11 are commodity (directly relating to EEPs) or hazard specific regulations which relate to EEPs. General food safety laws relating to all food, POAO generally or general feed controls have been excluded in order minimise the list and keep only those specifically relevant to EEPs.
6. Conclusions
This risk profile identifies and characterises the main hazards associated with shell eggs and egg products (EEPs) from domestic poultry species imported into the United Kingdom.
EEPs are consumed widely across the UK population, with over half of those surveyed consuming them; this is predominantly due to the inclusion of EEPs in a large number of products as well as the consumption of whole eggs. Infants are the highest chronic and acute consumers of eggs. Across all population subgroups, 97.5th percentile consumers eat the equivalent of 1-2 eggs per day.
The UK is active in the global trade of EEPs, exporting an average of 19,689 tonnes per year between 2016 and 2022. The largest importer of UK EEPs is the Netherlands, importing around 36% of the total. The UK imports an average of around 65,592 tonnes per year, with around 56% of these imported from the Netherlands. Of all imported EEPs approximately 70% are egg products rather than whole eggs.
Hazard identification
In the hazard identification phase, a range of over 100 individual hazards in EEPs was identified in the literature review and through incidents and alerts data. The hazards were attributed to one of 13 groups, encompassing allergens, chemical, microbiological, physical and radiological hazards. From this, the hazards for characterisation were shortlisted to 22 hazards or hazard groups using inclusion criteria and expert judgement.
No allergens, food additives, radiological contaminants or microplastics were characterised. For food additives this was because the those detected were approved in the UK or there was very limited evidence of presence in eggs. For allergens, microplastics and radiological contaminants there was no evidence that there was a specific consumer health risk relating to EEPs.
Microbiological hazard characterisation
Three microbiological hazards have been characterised in EEPs, Campylobacter spp, Listeria monocytogenes and non-typhoidal Salmonella (Salmonella Enteriditis & Salmonella Typhimurium). Salmonella Enteritidis was by far the most frequently reported and implicated in alerts and incidents across all of the hazards considered in this profile. As enteric pathogens, the primary exposure routes for Campylobacter and Salmonella are through contaminated feed, water, or from exposure to environmental sources. Listeria monocytogenes can also contaminate via this route, however it also has the ability to persist in the environment, and can establish a presence in a manufacturing environment, where it can contaminate egg products.
Human health effects of these pathogens vary depending on exposure and consumer group. If these pathogens are found to be present in eggs, there may be a concern for consumer health and further assessment would be required.
Chemical hazard characterisation
The agricultural contaminants, aflatoxins, OTA and PAs were characterised in EEPs; they can potentially contaminate feed resulting in poultry exposure. Aflatoxin and OTA formation in feed is linked to growth in a hot and humid climate. Conversely, PA concentrations are expected to be higher where nutrient availability is low and there is high soil moisture. Legal limits are in place for aflatoxins in feed in GB and NI.
Several environmental contaminants were identified in EEPs. POPs such as dioxins and dioxin-like substances, PCBs, PFAS and PCNs were taken forward for characterisation. POPs can bioaccumulate and can contaminate plants and soil, and hence can be transferred to poultry via animal feed. Proximity to anthropogenic sources of these contaminants is likely to impact the levels detected in eggs. Levels of dioxins and dioxin-like substances and PCBs are controlled in eggs in GB and NI, and levels of PFAS are controlled in eggs in NI.
Melamine was also characterised. Melamine can be present in food due to use in FCMs and sanitising solutions. It can also be present in veterinary medicine residues, as a feed additive impurity or through illegal use. It is most likely present in EEPs due to transfer via feed, although contamination through other routes is possible. Levels of melamine are controlled in food and feed in GB and NI.
A range of metals may contaminate EEPs due to their presence in the environment and emissions from industrial processes. Proximity to anthropogenic sources of metals is likely to impact the levels detected in eggs. Copper has been detected in eggs primarily due to its use as a pesticide.
Pesticide, biocide, feed additive and VMP residues have been detected in eggs at levels exceeding the respective MRL for certain specific substances. Residues above the MRL may result from the misuse of approved substances or illegal use of banned or non-authorised-substances that used in layer farms or egg processing environments.
The aforementioned chemicals potentially pose a human health concern; the potential adverse effects depend on their toxicity and the levels present. Where they are detected in EEPs, either at levels exceeding a legal limit (where one is in place), or at any level associated with potential human health concerns, a risk assessment would be required.
Mitigation and controls
Key mitigations for the majority of the chemical hazards in scope include the location of the poultry farm away from areas of high environmental contamination. Feed and water monitoring ensures that they meet the relevant guidelines and legislation and are compliant with legal limits for contaminants such as pesticides and veterinary medicines. There are no mitigations for chemical hazards once in the egg.
For microbiological hazards, key mitigations include applying HACCP controls, vaccination and restricting exposure to wild birds. Unlike chemical hazards, there is scope to mitigate microbiological hazards in EEPs via cooking or pasteurisation. Measures to prevent cross-contamination at all stages of production and in particular during processing are also important.
Extensive regulations relating to EEPs are in place in GB and NI. These include overarching regulations predominantly concerned with general criteria such as rules for public health requirements (including hygiene, microbiological criteria, contaminants regulations, additives regulations, use of antimicrobials). Many of these regulations are applicable to POAO rather than eggs specifically. In addition, there are regulations and legislation specific to the majority of the hazards identified in EEPs.
7. Uncertainties and Knowledge gaps
The level of uncertainty was estimated according to the categorisation defined in the ACMSF report on risk representation (ACMSF, 2020). Justifications for the uncertainty assigned to each area of the risk profile are provided in Table 12.
Knowledge gaps were identified during the review of information for this risk profile. As well as the uncertainty and justification, this section includes notes on identified knowledge gaps and discussion on their potential impact. Where appropriate, the impact of a knowledge gap is discussed as low, medium or high with justification. This is necessarily subjective but accounts for the scope of this risk profile and the levels of uncertainty.
Article updated on the 27th of November 2024.

_.png)




_.png)