Executive summary
Since the first confirmed case on 25th March 2024, Highly Pathogenic Avian Influenza A (H5N1) clade 2.3.4.4b genotype B3.13 has been detected in dairy cattle across the United States. The American Food and Drug Administration (FDA) in collaboration with states tested unpasteurised milk samples from bulk tanks, finding 57.5% of 275 samples positive for viral RNA. Of these, 25% contained infectious virus, with titres ranging from 1.3 to 6.3 Log10 EID50/mL and a median titre of 3.5 Log10 EID50/mL. During the outbreak, the FDA also found viral RNA in about 20% of tested dairy products from retail stores. However, no infectious virus was detected in any of the samples. Furthermore, two US studies of cattle infected with H5N1 have shown 0.5-1% of muscle samples have viral RNA. This was reported to be at very low levels and no virus was detected in muscle associated with the “usual” cuts and joints of beef. In beef offal, H5N1 has been detected in the lung at a titre of 2.8 Log10 TCID50/mL and mammary glands at 7.3-7.8 Log10 TCID50/mL.
This risk assessment is an update to a previous risk assessment (Rapid Risk Assessment: Risk to UK consumers from Highly Pathogenic Avian Influenza (HPAI) H5N1 B3.13 in US dairy products | Food Standards Agency) to take into account the latest information and evidence. This assessment has also expanded the scope to include the risk from beef and other meat products exported to the UK from the USA. Therefore, this risk assessment will evaluate the risk of H5N1 to UK consumers from the consumption of pasteurised milk and pasteurised dairy products; raw/unpasteurised milk and raw/unpasteurised dairy products; colostrum and colostrum-based products; fresh meat including offal (i.e. meat from the bone); and minced meat imported from the US.
The UK imports milk and dairy products from the US (1,066,214Kg from 1st January to 7th November 2024) as well as beef (18,396Kg from January to November 2024).
The available evidence suggests that the virus is susceptible to heat treatment and normal pasteurisation methods (Low Temperature Long Time and High Temperature Short Time) have been shown to reduce live infective virus in milk by 4.44 log to more than 6 log. Since the previous risk assessment, APHIS have confirmed that all US exported dairy products are pasteurised. We assess the probability of individuals being exposed to an infectious dose of H5N1 virus from pasteurised milk, pasteurised colostrum or dairy products made from pasteurised milk to be Negligible with a Medium level of uncertainty
Studies of cattle infected with H5N1 found low levels of viral RNA in under 1% of samples, and no virus was detected in muscle associated with the “usual” cuts and joints of beef. In beef offal, H5N1 has been detected in the lung and mammary glands, in the latter at high levels. Imported US beef and offal accounts for a small proportion consumed in the UK (0.0024%). Additionally, cooking temperatures have been shown to reduce the infectivity of the virus. We therefore assess the probability that UK consumers will receive infectious exposures to influenza of avian origin via imported US beef and offal to be Negligible with a Medium level of uncertainty.
In the event of infection, the severity of detriment has been determined as Low. This is due to the fact that the only confirmed human cases of HPAI H5N1 (clade 2.3.4.4b genotype B3.13) infection, to date, have been mild cases in American farm workers who were in close contact with infected cattle. However, this is given with a Medium level of uncertainty as foodborne exposure has not been confirmed and it is unknown whether severity of illness will be different compared to other routes of exposure such as inhalation.
Statement of purpose
This assessment updates a previous risk assessment carried out in April/May 2024 on the risk to UK consumers from Highly Pathogenic Avian Influenza (HPAI) H5N1 B3.13 in US dairy products (Rapid Risk Assessment: Risk to UK consumers from Highly Pathogenic Avian Influenza (HPAI) H5N1 B3.13 in US dairy products | Food Standards Agency). It takes into account new information and scientific evidence which has emerged since, as well as to expand the scope of the assessment to include the risk from beef and other meat products exported to the UK from the USA. Products within scope of this assessment are therefore:
-
pasteurised milk and pasteurised dairy products;
-
raw/unpasteurised milk and raw/unpasteurised dairy products;
-
colostrum and colostrum-based products;
-
fresh meat including offal (i.e. meat from the bone);
-
minced meat.
Risk question
Given that Highly Pathogenic Avian Influenza virus (H5N1) has been found in raw milk and that fragments of RNA have been found in pasteurised milk in the US, what is the risk of H5N1 to UK consumers from the consumption of the dairy and beef products listed above.
Background
Since the first confirmed case on 25th March 2024, a number of dairy herds in the US were reported as exhibiting clinical signs including inappetence and milk drop. 10-15% of animals on affected farms showed clinical signs, with minimal deaths. At sites in Texas, deaths of wild birds (pigeons, blackbirds, and grackles) and domestic cats, assumed to have consumed unpasteurised milk, were also reported (APHIS, 2024a).
Although regional diagnostic labs were initially unable to identify a cause, samples received at the Iowa State University Diagnostic Laboratory in Ames on 24 March 2024 (Texas) and 25 March 2024 (Kansas) were found to be positive for influenza A virus (IAV) by PCR. A press release was issued on 25 March 2024 (APHIS, 2024a) and an early-access manuscript fully describing these results was published on 29 April 2024 (Burrough, et al., 2024). The first confirmed case of this outbreak was on the 25th March 2024, sequence analysis raises the possible crossover to cattle could have occurred earlier (Science.org, 2024)
On 25 April 2024, the FDA reported that PCR testing on samples from a nationally representative commercial milk sampling study found influenza virus RNA in around 20% of retail samples tested, although the number of samples tested was not given (FDA, 2024). On 26 April 2024 the FDA reported some preliminary results suggesting that pasteurisation is effective at eliminating infectious HPAI from milk (FDA, 2024).
Since its detection the outbreak has continued to spread. Several routes have been proposed as possible mechanisms for spread between farms and to other states (Tomlinson, 2024). These include the movement of infected animals, the movement of contaminated milking equipment, and spread via wild birds (Burrough, et al., 2024). There are currently no national restrictions on cattle movement in response to this outbreak, however in April 2024 the US passed a federal order requiring mandatory testing and reporting for HPAI for the interstate Movement of dairy cattle.
The Animal and Plant Health Inspection Service (APHIS) has established a page linking to the latest updates on the situation at Highly Pathogenic Avian Influenza (HPAI) Detections in Livestock | Animal and Plant Health Inspection Service (usda.gov), which also includes a regularly updated map of states that have detected the current strain of influenza in livestock.
Hazard identification
The hazard considered for this assessment is an influenza A virus of avian origin classified as B3.13. Influenza viruses are negative-sense, single-stranded, enveloped RNA viruses classified in the family Orthomyxoviridae, and 80-120nm in diameter. The H5N1, a Eurasian/North American reassortment virus, implicated in the USA dairy cattle outbreak has never been detected in the UK or Europe. In addition to this, no other Eurasian-North American reassortment have been recorded in the UK or Europe, despite detections of these in North America since 2014 (HAIRS, 2024).
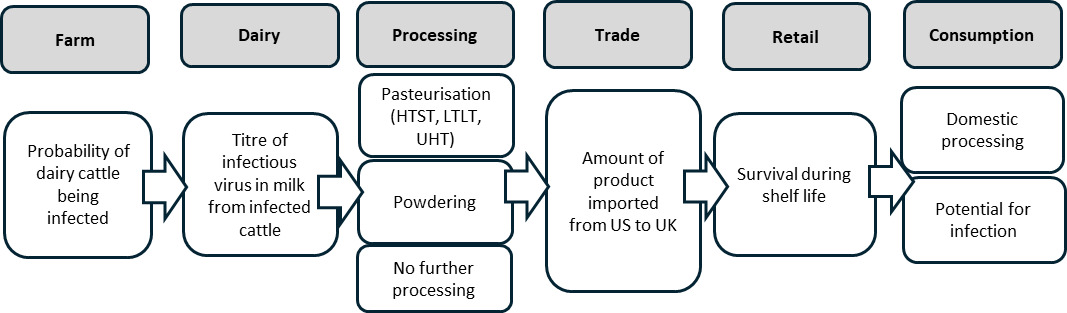
Exposure assessment (Dairy)
Risk Pathway
Farm: probability of dairy cattle being infected with B3.13
The HPAI outbreak has impacted dairy cattle herds across the United States. As of November 8, 2024, the USDA has confirmed infections in dairy herds across 15 states. The number of herds confirmed infected varies by state, with California reporting the most. California reported its first cases on August 30, 2024, and has since recorded a total of 277 positive cases, with 172 occurring between October 9 and November 8, 2024 (30-day period). California, Idaho, and Utah were the only states to report any cases between October and November 2024.
Investigation and reporting is a mixture of state and federal level, with surveillance and sampling effort and approaches varying by state, so the true size of the outbreak is difficult to estimate.
Two studies of infected dairy herds during the current outbreak estimated the number of cattle showing clinical signs at any one time to be around 10-15% (Burrough, et al., 2024) and 3-20% (Caserta et al., 2024). The true incidence of infection is more difficult to estimate because not all infected cattle will show clinical signs, and subclinical infected animals may also shed infectious virus in milk. A study by Michigan State University, using elevated body temperature and decreased rumination as indicators of infection, estimated that around 40% of the herd became infected over the course of the outbreak.
Based on the daily case reports observed in the first study, the incidence of clinical signs peaked 4–6 days after the first was observed, and new reports began to decline after 10–14 days. After this period, most animals were slowly returned to regular milking (Burrough, et al., 2024).
On affected Texan farms, affected animals exhibited reduced feed intake and rumination, and an abrupt drop in milk production. Milk from the most affected cows had a thickened, creamy yellow appearance. Figure 2 shows an example of milk from affected cattle. Some milk shows evidence of curdling (Caserta et al., 2024). Somatic cell count (SCC) is used an indicator of milk quality. SCC becomes present in increasing numbers in milk usually as an immune response to a mastitis-causing pathogen. Rodriguez et al. showed that SCC counts in the bulk tank more than doubled during a HPAI herd infection and took about 2 months to return to pre-infection levels after the onset of clinical signs (Rodriguez et al., 2024).
USDA guidance states that milk from clinically affected animals should be discarded according to state and local regulations, preventing contaminated milk from entering the food chain (APHIS, 2024a). The milk from infected cattle is thicker and colostrum-like (USDA, 2024). The FDA recommends that producers discard milk from clinically affected cows (Zack, 2024). The FDA and USDA have indicated that they believe the US commercial milk supply to be safe both because milk from clinically affected cows should be diverted or destroyed and because of the effects of pasteurisation (FDA, 2024).
Milk presents a thickened appearance and colouration varying from yellowish to pink/brown colour. Curdling of milk is visible in some samples (Caserta et al., 2024). In summary, the number of US farms currently infected is uncertain but infection has been confirmed on 864 dairy herds to date (APHIS, 2024a) and this is likely underestimated. The number of cattle infected on affected farms is also uncertain due to limited data and the unknown proportion of subclinical infections.
Dairy: titre of virus in milk from infected cattle
Unpasteurised milk shows high levels of viral nucleic acid, meaning a low Ct (cycle threshold) value in a PCR test. The lowest Ct value reported is 12.3 (Burrough, et al., 2024). The presence of viral nucleic acid alone does not necessarily reflect the presence of infectious virus, although the presence of some live virus in these raw milk samples has been confirmed. In a worst-case scenario, the quantity of viral RNA that would result in Ct value of 10 would be around 108.5 Log10 infectious virus particles per ml of milk.
However, we cannot rule out the possibility of milk from subclinical infected cattle entering the food chain without detection, or for milk from clinically affected cattle to entering the food chain through error or non-compliance with USDA guidance. Sampling of unpasteurised bulk milk supports this. The FDA, in collaboration with four states, tested unpasteurised milk samples from bulk tanks (capacity to hold milk from 600 to 700 cows) collecting from both affected and unaffected farms in regions with confirmed infections. These samples are collected routinely as part of procedures under the Pasteurised Milk Ordinance (see next section). Out of 275 raw milk samples tested using quantitative reverse transcription PCR, 158 (57.5%) were positive. Of the positive samples, 39 (25% of PCR-positive, or 14% of the total) contained infectious virus, with titres ranging from 1.3 to 6.3 Log10 EID50/mL, and a median titre of 3.5 Log10 EID50/mL. Authors noted that there was no strong correlation between the viral titre estimated from the PCR result and the amount of viable virus detected (R2 = 0.37) (Spackman et al., 2024).
Commercial processing: effects on virus
Processing used in the production of dairy products
Since 1987 the FDA has encouraged participation in the voluntary Grade “A” Program; nearly all (99%) of milk produced in the US comes from farms that participate in this programme and follow the Pasteurized Milk Ordinance (PMO), which requires the pasteurisation of milk and the prevention of milk from sick cattle from entering the food supply (ASTHO, 2024). In addition, since 1987 the FDA has required dairy products moving interstate to either be pasteurised or – in the case of cheese – aged for a minimum of 60 days. The FDA have confirmed that international export is covered by interstate movement and falls under the same requirements. All imports into the UK from the US should be pasteurised.
However, FDA regulations state that cheese made with unpasteurised milk can be sold in interstate and international commerce for human consumption, with the stipulation that it must be aged for 60 days. Therefore, the only dairy product for human consumption, exported to the UK from the US that may not have undergone pasteurisation is aged cheese.
Pasteurisation
Pasteurisation is a process that involves heating milk to a high temperature followed by rapid cooling before it is bottled or packaged, to ensure safety to the consumer by eliminating harmful bacteria present in the milk as well as extend shelf life. Different temperature-time combinations are permitted, with the holding time being that required to achieve a specified reduction in the titre of the most heat-resistant microorganism typically found in the foodstuff at the indicated temperature. For milk, this is a 5 Log reduction in the numbers of Coxiella burnetii (Codex & FAO, 2011).
In the UK, milk is normally pasteurised using one of the following three methods:
High Temperature Short Time (HTST) is a continuous method in which milk is heated to 71.7°C for 15 seconds. It is used for large scale processing of liquid dairy products and is commonly used for drinking milk.
Low Temperature Long Time (LTLT) is a batch method in which milk is heated to 63°C for 30 minutes. It is commonly used for small batch pasteurisation, for example as part of the on-farm production of dairy products such as ice cream.
Ultra-High Temperature (UHT), which involves heating the milk to at least 138°C for at least 2 seconds.
In addition, US regulations permit three further temperature-time combinations: 89°C for a minimum of one second, 96°C for a minimum of 0.05 seconds, or 100°C for a minimum of 0.01 seconds (IDFA, 2024).
To evaluate the effectiveness of pasteurisation in mitigating the risk of virus contamination in milk, a study spiked milk with 6.3 Log10 EID50/mL of clade 2.3.4.4b H5N1 virus (Alkie et al., 2025). This is the highest titre found in raw bulk milk tank testing (Miller, 2022; Spackman et al., 2024). After LTLT pasteurisation no infectious virus could be detected (>6 Log reduction). After HTST pasteurisation no infectious virus could be detected in seven out of eight experimental replicates (>6 Log reduction). In one replicate, a 4.44 Log virus reduction was achieved, which would be sufficient to eliminate the typical viral quantities detected in bulk milk from affected areas. There are no data available on whether the other permitted temperature-time combinations are effective.
In May 2024, Schafers et al., published findings on the effectiveness of pasteurisation on inactivating influenza A viruses in cow’s milk. They found that commercial pasteurisation conditions, LTLT: 62.5°C for at least 30 minutes, and HTST: 72°C for at least 15 seconds, rapidly inactivated influenza A type viruses and reduced the titre of infectious virus from ~107 PFU/mL to below the LoD, 33 PFU/ml (~5-6 Log reduction) in all instances. Infectivity of HPAI H5N1, as assessed in egg haemagglutination assay found that while viral genetic material remained detectable after pasteurisation, no infectious virus was recovered from pasteurised milk (Schafers et al., 2024).
In July 2024, Franziska Kaiser, in a letter to the editor of the NEJM reported similar findings using raw cow’s milk spiked with the virus to 106 TCID50/mL HPAI A(H5N1) virus A. At 63°C they found that virus inactivated from 106 TCID50/mL to undetectable levels within 2 minutes. (Estimated half-life of infectious virus: 4.5 seconds). At 72°C, the virus titre decreased from 105 TCID50/mL to 101 TCID50/mL within 5 seconds. Very low titres (<101 TCID50/mL) were detected until 20 seconds; no viable virus was found at later time points (Kaiser et al., 2024).
In June 2024, Cui et al., published findings on the thermal stability of Influenza A viruses, including H5 clade 2.3.4.4b viruses. They found that submitting milk containing virus at titres of ~107 EID50/mL to 63°C for 30 minutes or 72°C for 15 seconds achieved a complete inactivation of H5N1 viruses, corresponding to a reduction of around 106.5 EID50/mL as measured in an embryonated chicken egg viral assay (Cui et al., 2024).
Caceres et al., investigated the stability of the virus in commercially available pasteurised whole milk, spiked with 8 Log10/mL virus, under various thermal conditions. Heat treatment at 63°C for 30 minutes effectively reduced viral viability below the limit of detection (1 Log10 TCID50/mL) representing at least a 7 Log reduction in viral titre. At 72°C for 20 seconds, the reduction in viral titre was inversely proportional to the volume of sample that was heat treated. Significant reductions in virus were observed in the smallest volume (2 µl, 6 to 7 Log reduction) but little or no reduction was seen in the largest of the sample volumes tested (200 µl, <1 Log reduction). Treatment at 91°C for 20 seconds resulted in larger reductions in viral titre than 72°C for 20 seconds, but again the reduction was inversely proportional to sample volume. It is unclear why these findings are in contradiction to other similar studies or why efficacy of pasteurisation is inversely related to sample size (Caceres et al., 2024).
Powdered milk products
There are three common methods for producing powdered milk (Miller, 2022). These consist of:
- Spray Drying - milk is first flash-pasteurised to kill any bacteria. It’s then reduced to around 50% of its original mass by steaming, separating the vapour from the powder and condensing it to a liquid which is removed. The milk is then sprayed into a drying chamber, exposing it to hot air. The liquid milk droplets quickly evaporate, leaving behind a dry powder.
-
Drum Drying - a faster method that involves passing the milk over a thin film on a heated drum which steams the milk, leaving solids behind which are extracted and ground into a fine powder. Of the three methods listed here, drum drying is the least used as it can caramelise the milk.
-
Freeze Drying - freeze drying is complex and expensive and involves slowly freezing the milk at -50°C to -80°C. Milk is then subjected to low heat and low pressures in a partial vacuum, which helps a process called sublimation turning ice from solid state to gas. The gas is then condensed and collected, leaving behind a dry powder.
It is highly likely that the first two methods would inactivate any virus present in milk, although the effect of the final method is less certain.
On 26 April 2024 the FDA published a statement that PCR testing of “several” powdered infant and toddler formula samples came back negative (FDA, 2024). The statement does not specify the number of samples tested (although they were a subset of “an initial limited set of geographically targeted samples” collected as part of a national commercial milk sampling study in coordination with UDSA). The statement also does not state which of the aforementioned processing methods was used.
Unpasteurised products
Even if unpasteurised milk is used to make cheese, reheating of the curd is used in the production processes of many such cheeses, which is likely to reduce virus levels. There have been very few studies assessing the survival of virus in cheese. Lang et al. (2024) assessed the survival of H5N1 clade 2.3.4.4b (H5N1-22) and H5N1 clade 2.2.2, (H5N1-06) in a variety of dairy products. They made their own cheese, using virus spiked milk at a concentration of 104 - 104.5 Log10 pfu/mL. As part of their cheese making process the milk was heated to 80-85°C. No viable virus was detected in either curds or whey, although viral RNA was found in the curds but not in the whey. In an older study, Cliver, (1973) investigated the survival of Influenza A in cheddar cheese. The initial titre in the milk was 7.5 x 107pfu/g. Influenza A was found to be below the limit of detection in the whey and in the curd after pressing (i.e. <1.3 x 103 pfu/g) - a reduction of over 4 Log. The stability of virus in the curd during aging was not studied because the virus was already below a measurable level when ageing began.
The study by Lang et al. (2024) also investigated the survival of H5N1 viruses in other homemade dairy products. They found viral RNA in yoghurt samples, although no viable virus was detected. The authors suggest that the acidic pH contributes to the loss of virus infectivity. They also detected viral RNA and viable virus was in fresh milk. This may be due to the antimicrobial and antiviral properties of whey components, such as lactoferrin and lactalbumin, or may indicate that virus attaches to other proteins in milk such as casein more readily than to whey protein.
A retail sample survey (Table 1) tested a variety of dairy products from multiple retail locations for live virus and viral RNA. This sampling was conducted between 18 June and 31 July 2024. Out of 167 samples, none tested positive for viable H5N1, while 17% (29 samples) were positive for viral RNA. Skim milk, heavy cream, cream cheese and aged raw cheese had no viral RNA detected. Viral RNA was detectable in a small to moderate proportion of whole, 1%, and 2% milk (4.3% to 18.3%), and could be detected in 14.3-16.7% of cheddar and processed cheese. Viral RNA was detected in a higher proportion of mozzarella cheese, butter and ice cream (40.9% to 50%), although the number of samples of each product tested was small. The non-detection of viral RNA in products such as raw milk is likely due to the milk not being contaminated, rather than the result of processing, considering the other results presented above. The production date of aged cheese is also unknown; however, aged cheese available at retail is likely to have been produced prior to the outbreak in cattle. On 21 November 2024 the California Department of Public Health announced a recall of whole raw milk from one particular batch being sold as raw milk at retail, after testing had confirmed the virus was present.
Trade: amount of product imported from US to UK
So far in 2024 (1 January to 7 November) a total of 1,066,214kg (Table 2) of milk and dairy product has been imported to the UK from the USA. Dairy imports to date from non-EU countries in quarter 3 of 2024 are 5,000,000 kg (July – Sept). Assuming imports stay constant over each quarter of 2024, it assumed that non-EU imports to the UK will equate to 20,000,000 kg in 2024. Therefore, US milk and dairy imports make up approximately 1/20 of all non-EU milk and dairy imports (AHDB, 2024b). 812,160kg (76.2%) of this was dried, and a further 168,564kg (15.8%) was for ice cream and other edible ice products. Whey products account for 52,586kg (4.9%), while 19 consignments of ‘other’ total 24,183kg (2.3%). Grated or powdered cheese accounts for 72kg (0.007%), whereas ‘other’ cheese accounts for 8,493kg (0.80%). However, fresh (unripened or uncured) cheese had 1 consignment, but the total net weight was not reported. Additionally, frozen milk and cream (not concentrated) accounts for 2kg of import and butter accounts for 154kg (0.014%) (Pers. Comms. Imports Strategy Team (FSA), personal communication, 2024a).
More than 99% of legal dairy products imported into the UK will be pasteurised, and we can presume the amount of virus will be significantly reduced by between a 4.4 and >6 Log (uncertainty). Less than 1% (other cheese) may be unpasteurised but should be aged.
Data from Statista suggest that 14.9 million litres of milk was produced in the UK in 2022/23 (Statista, 2024a) and the Agriculture and Horticulture Development Board (AHDB) reports that 5,626,281,800 kg of dairy produce were purchased at retail in the UK in 2024 (AHDB, 2024a). Therefore, US imports of dairy produce make up a small proportion of the market.
In 2023, the volumes were approximately 2.45 times higher (2,617,518kg) than 2024 (1st January to 7th November) and included small amounts of (largest first) cheese and curd, whey, grated/powdered cheese, “other” cheese, butter, blue cheese, processed cheese, and milk/cream (Pers. Comms. Imports Strategy Team (FSA), personal communication, 2024a). The reason for the higher import volume is unclear (uncertainty)
Under certain specific conditions virus can remain infectious during freeze-drying. Although it is highly unlikely that conditions during commercial dried milk powder freeze-drying would be suitable, validation of this assumption is recommended. APHIS have confirmed with the FSA that "interstate commerce of raw milk for human consumption is prohibited under the FDA’s authority (see US Code of Federal Regulations at 21 CFR 1240.61). Milk products, like dried milk powder, are pasteurized for introduction into interstate (and therefore international) commerce" (APHIS Pers. Comm, 2024). Regarding imports to the UK, FSA trade imports team have confirmed that the GB would require dairy establishments to be approved, so where any processing of diary product take places which are exported to GB, these establishments would need to be listed with the UK Office, a department of Defra. Listing or approval to export to GB means that they meet our food safety requirements, so those that appear in 852/2004 and 853/2004 and any other GB specific dairy requirement (Pers. Comm. FSA imports strategy team, personal communication, 2024).
There is significant uncertainty around the amount of colostrum recently imported into the UK, its uses, and any processing undertaken. Defra colleagues located a single consignment of “colostrum” imported in December 2023 of size 2.2kg. Colostrum is consumed as a supplement by athletes and bodybuilders. Colostrum can be chilled, frozen, dried and will have Health Certificates stating the process used in temperature criteria. It is not likely to be raw through legitimate channels but raw colostrum products that are consumed in the US so could arrive in to the UK via non-legitimate routes (Pers. Comms. Imports Strategy Team (FSA), personal communication, 2024b).
Retail: survival during shelf life
Dairy products such as milk and cheese are often stored at chilled temperatures. A study by Nooruzzaman et al., (2024) stored milk from infected cows at 4°C (refrigerator temperature) and observed a reduction in infectious virus from 5.97 Log10TCID50/mL on day 0 to 2.05 Log10TCID50/mL after 42 days, with virus still detectable after 56 days. Therefore, virus present in milk or dairy products may persist at refrigeration temperatures for weeks or months. Virus which survives the freeze-drying process can persist very well at ambient storage temperatures.
Surveillance data indicates that HPAI viruses, such as H5N1, can persist in frozen duck meat (Chmielewski et al., 2011). This persistence suggests that virus which is not inactivated during the production of frozen dairy products such as ice cream could persist for prolonged periods. However, unpasteurised dairy products including ice cream cannot be exported to the UK.
Consumption
Domestic processing
Consumers often process dairy products in various ways, such as grilling or incorporating them into sauces. These methods typically involve heating the products to high temperatures, which is likely to inactivate the virus. However, these products are also often consumed without further processing, so to yield a conservative estimate of risk we do not consider consumer processing guaranteed to reduce the probability of exposure.
Consumption of dairy products
Consumption of unpasteurised dairy products with viable virus is a potential exposure risk. Data from the National Diet and Nutrition Survey (NDNS, 2021) indicates that cow’s milk is the dairy product consumed in the highest amount per portion (compared to cheese, milk powder, whey powder, yoghurt and ice cream). Consumption of cow’s milk varies across different age groups. Individuals under 65 years of age consume on average 80 to 150 grams of cow’s milk per serving. The average serving by age group: Infants (4-18 months)-110 grams, Toddlers (1.5-3 years)-150 grams, Children (4-10 years)-150 grams, older children (11-18 years)- 140 grams and Adults (19-64 years)-80 grams. The elderly (≥65 years) have lower mean intakes of cow’s milk (69 grams), indicating a reduced exposure risk. However, the lack of export health certificates for unpasteurised products means this is a very low (at most) likelihood exposure pathway for products on the shelf in the UK.
Exposure assessment (Beef)
Risk Pathway (US Beef)
Farm: probability of cattle entering food chain being infected
Although most beef entering the food chain in the USA comes from beef herds, spent dairy cattle may also be used, particularly for ground beef. However, it is assumed that beef that will be sold internationally is likely to be of high quality i.e. cattle where antibiotics and hormone use have not been given. The section above describes the probability of dairy cattle being infected.
Two separate FSIS studies have investigated the potential presence of H5N1 viral RNA in muscle tissue from dairy cattle as part of a programme of surveillance in states where virus was known to be circulating, each with a distinct focus and sampling approach. The first focused on condemned animals at slaughter facilities, where one out of 109 muscle samples tested positive for H5N1 viral RNA at very low levels. The second, part of the FSIS’s broader surveillance program launched in September 2024, involved routine testing of culled dairy cows regardless of clinical signs. Only one muscle sample tested positive for H5N1 viral RNA (APHIS, 2024a). These findings suggest that the low detection rates likely reflect a low prevalence of infection among sampled animals. However, because the sampling was not targeted specifically at farms or regions where virus was known to be circulating, the prevalence on infected farms is likely to be higher. Additional sampling from one of the carcases, including several different types of muscle tissues that correspond to common retail cuts of meat, did not detect infectious virus or viral particles. In beef offal, H5N1 has been detected in the lung at a titre of 2.8 Log10 TCID50/mL and mammary glands at 7.3-7.8 Log10 TCID50/mL. In the UK there are robust systems at slaughterhouses to ensure that animals that are not fit for consumption are detected and removed from the food chain. It is likely that any animal with clinical signs, and thus with a high viral load, would be detected via these routes and not enter the food chain.
Beef: titre of virus in beef (fresh meat, minced and offal) from infected cattle
Although limited information is available for influenza virus in cattle, we know that in infected birds the tissue distribution of influenza virus varies by strain and species. However, the proportion of cattle infections in which muscle tissue contains virus appears to be low. Additional sampling from the carcase described in the FSIS study above, including several different types of muscle tissues that correspond to common retail cuts of meat, did not detect H5N1 influenza A virus (APHIS, 2024b). We are not aware of any studies of infected beef cattle or surveillance of beef herds for H5N1 to date (uncertainty).
The picture for offal may be more complicated. A study by Caserta et al. (2024) studied samples from dairy cattle from nine affected farms across four states in the USA. Results from rRT–PCR on tissues collected from three affected cows revealed the presence of viral RNA in the lung, small intestine, supramammary lymph nodes and mammary glands, with the highest viral loads in the mammary glands. Samples of ground beef derived from dairy herds that have tested positive for H5N1, which were condemned at select FSIS-inspected slaughterhouses, have been tested at retail. No viral RNA was detected by PCR (APHIS, 2024b). None of the studies above have quantified the virus (uncertainty).
Commercial processing: effects on virus
Ready meals
It is possible that beef imported from the US could be used in the production of ready meals after import. This production is likely to involve processing that will significantly reduce the levels of infectious virus present, as Regulation No 852/2004 on the hygiene of foodstuffs requires food business only place safe food on the market. There is no legislation that instructs businesses how to achieve safety of the food they produce, but food safety is warranted by the requirement for all food business operators (FBOs) to have specific HACCP-based plans and procedures in their food production premises. It is assumed that FBOs producing cooked foods, will adhere to these regulatory requirements (uncertainty).
Trade: amount of product imported from US to UK
Data from Statista suggest that 11.34 kg of beef/veal were consumed per capita in the UK in 2022 and is estimated to stay at this level for the next 3 years (Statista, 2024b). The population of the UK in 2023 was 68,265,200 (ONS, 2024) which suggests around 774,000,000 kg of beef is eaten per year.
Beef imported to the UK from the US is subject to strict tariffs and production conditions, such as no hormone use (Trade tariff, 2024). The total beef imported into the UK from the US was 18,396Kg from January to November 2024 (Table 3), which is lower than beef product imported in 2023 (104,296 kg). Beef imported from the US therefore makes up a tiny fraction (0.0024%) of all beef consumed in the UK. Beef imports are included within 4 CN tariff codes which encompasses frozen, chilled, or ambient products.
Imports of beef from the US have significantly decreased. The reasons for the reduction in import volume is not clear but could be due to the tariff system and conditions imposed on imports (uncertainty).
Retail: survival during shelf life
Beef and products containing beef are normally stored at chilled or frozen temperatures. Although there are no specific studies of this strain in beef or beef products, viruses survive longer at lower temperatures, and studies using poultry meat confirm that H5N1 is able to survive in muscle tissue for extended periods. In a small study by Yamamoto, Nakamura and Mase (2017) demonstrated that H5N1 could still be detected after 160 days at 4°C in poultry muscle, although viral titres decreased by between 2.0 and 3.7 Log10 EID50/mL. By 160 days there was no detectable virus. In the same study, they showed that virus could still be detected in poultry liver kept at 4°C for at least 20 days, although the titre decreased between 0.7 and 5.3 Log10 EID50/ml and the virus was undetectable in all samples by day 30. At 20°C, equivalent to ambient temperature at which products such as beef jerky would be kept, virus in poultry muscle was detectable for up to 20 days but titre declined by between 1.8 and 4.5 Log10 EID50/ml and was undetectable by day 30.
Consumption
Domestic processing
Luchansky et al. (2024) experimentally measured the inactivation of H5N1 in beef burgers (“patties”) during cooking. They used 300g of meat (3 times higher than a “normal” burger) and spiked them with 5.6 Log10 EID50 per 300g H5N1 influenza A virus. Temperature was continuously monitored at 5-s intervals until the target internal temperature of the meat was achieved. Once achieved the meat was removed from the grill and within 2 minutes was placed in a water bath. Cooking to an internal temperature of 71.1°C (considered “well done” in this study and the FSIS recommended cooking temperature) took an average of 24 minutes and resulted in no virus being detected (at least 5.6 Log reduction). Even at a lower temperature of 62.8°C resulting in a “medium” cooked burger, cooked for an average of 22 minutes, no virus was detected either. They also considered LTTC burgers, cooking at 48.9°C which took around 15 minutes to reach temperature. Even at this low temperature a reduction in the titre of infectious virus from 5.6 ± 0.4 Log10 EID50/300g in uncooked burgers to 3.1 ± 0.4 Log10 EID50/300g (2.5 Log reduction).
Consumption of beef and offal
Data from the NDNS indicates that beef and beef products (such as mince, beef fat, ready-made beef burgers) are consumed in higher amounts per portion than beef offal. Adults aged 19-64 have the highest mean intake of beef with a serving size of 110g, followed by adults aged ≥65 years at 91g, then children aged 11 – 18 at 88g suggesting that these groups are at highest risk of exposure through consumption of contaminated meat. Children under 3 years have lowest mean serving size of beef at 39g per portion suggesting that they have the lowest risk of exposure to H5N1 via consumption of contaminated beef. Adults aged ≥65 years group consume the largest serving of beef offal at a mean portion size of 23g per person per serving. Across all age groups the mean serving size of beef is 77.g and beef offal 15.6g.
Therefore, coupled with the exceedingly small volumes of legitimate imports of beef meat and unprocessed products from the USA and the export health certificate requirements for approved farms, then the risk level is further reduced.
There are numerous supplements available on the market that contain beef products, which are powdered products and would be dried before being ground. We do not know what processing is involved in drying the products, some products on the market are keen to stress that “low temperature processing” has been used in the manufacture. The specific details of the processes used are unknown but freeze drying is discussed in the dairy section of this risk assessment and the effectiveness of such a process on the viability of H5N1 is uncertain. Examples of products with serving sizes are shown in Table 5. Serving sizes are small compared to general beef consumption.
Searches on commonly used websites such as Amazon show that US-produced supplements can be purchased in the UK. We do not know how many of these supplements are purchased in the UK per year (uncertainty). US-produced supplements exported may contain extracts that are not from cattle reared in the US (uncertainty). Marketing for these products is aimed at a particular demographic i.e. body builders and those wishing to follow a paleo diet. This limited data suggests the maximum consumed portion size is 3.6g per day (Table 5). We have no further data on the consumption of these beef product supplements (uncertainty).
Hazard characterisation
Due to its recent emergence, limited information specific to HPAI clade 2.3.4.4b genotype B3.13 is available, and hazard characterisation will necessarily draw upon evidence relating to other strains, which will be reflected in the uncertainty assigned to our assessment.
Risk of AI infection from food
Although by far the most common transmission pathway for influenza virus to infect humans is via aerosol and droplet infections, experimental infections suggest that animals such as birds can contain very high viral titres in their tissues and internal organs post infection (up to 108.0 Log10 EID50, Thomas and Swayne, 2007). There is anecdotal evidence that the consumption of the uncooked blood or poultry meat, gamebirds or wild birds has transmitted HPAI H5N1 virus to carnivorous animals, including tigers (Hu et al., 2016), leopards (Keawcharoen et al., 2004) domestic cats (Kuiken, 2004), domestic dogs (Songserm et al., 2006), stone martens, (WHO, 2024), ferrets (Bertran & Swayne, 2014) and lions (Chen et al., 2016).
Reports of human infection are very rare. There have been isolated reports of individuals becoming ill after consuming infected uncooked animal products; in 2005 a 27-year-old Vietnamese woman became ill after she drank duck blood as part of a local dish. However, in this instance, other routes of transmission could not be ruled out (IATP, 2005). In 2006, the European Food Safety Authority (EFSA) Scientific Panel on Biological Hazards considered the risk of infection to humans from contaminated poultry and eggs and concluded that “there was no epidemiological evidence to date that avian influenza can be transmitted to humans through consumption of food” (EFSA, 2006).
The infectious dose of AI for humans is unknown. Several studies have investigated oral and intranasal inoculation of AI in animals (O’Brien et al., 2021). A study by Bertran and Swayne in 2014 in which ferrets were exposed to different HPAI viruses (H5 and H7 subtypes) through consumption of infected chicken meat, showed that the dose of virus needed to infect ferrets through consumption (108.9-109.2 EID50) was much higher than via respiratory exposure (107 EID50) and varied with the virus strain (Bertran & Swayne, 2014). A 2012 study by Reperant et al., showed that intragastric inoculation of domestic cats at a level of 107.8 TCID50 resulted in fatal systemic infection (Reperant et al., 2011). In 2011, Shinya et al showed that the inoculation of hamsters with H5N1 directly into the digestive tract at a level of 107.1-107.3 TCID50 allowed the virus to enter the bloodstream through the digestive lymphatic system (Shinya et al., 2011). It is unclear to what extent these dosages represent a biologically relevant risk of oral transmission. At present, there are no well-documented cases of human AI infection from food, where respiratory exposure could be completely ruled out (O’Brien et al., 2021). Collectively however, these results suggest that the orally infectious dose for humans is unlikely to be much lower than 107 TCID50.
Avian influenza and human health
Avian influenza infections in humans can present a wide spectrum of disease manifestations, ranging from mild to severe. Mild cases often resemble common flu symptoms, including fever, cough, sore throat, and muscle aches. Some individuals may experience conjunctivitis, which is an inflammation of the eye. Symptoms typically appear 3 to 5 days after infection. In more severe cases, avian influenza can lead to serious respiratory complications. Patients may develop pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ failure. These severe manifestations can require hospitalization and intensive medical care (NHS, 2024). The progression to severe disease is influenced by factors such as the specific strain of the virus, the patient’s age, underlying health conditions, and the timeliness of medical intervention. In rare instances, avian influenza infections can result in fatal outcomes. Higher mortality rates are associated with certain highly pathogenic avian influenza (HPAI) strains, such as H5N1 and H7N9. Infected cases may be given antiviral medicine such as oseltamivir or zanamivir, which may help reduce the severity of disease, prevent complications and improve the chances of survival (Smith, 2010).
Human cases of HPAI H5N1 linked to the most recent outbreak in the US
In April 2024, the WHO was notified of a human case of AI H5N1 in Texas. The individual, exposed to infected dairy cattle, developed conjunctivitis. Tests confirmed AI H5N1 clade 2.3.4.4b. The genotype was classified as B3.13, which was the same genotype detected in dairy cattle in Texas. The patient recovered after treatment with oseltamivir and no further cases were linked to this incident. In May 2024, two human cases were reported in Michigan. These unrelated cases were both dairy workers exposed to cattle infected with AI H5N1 clade 2.3.4.4b genotype B3.13. One had conjunctivitis, the other mild respiratory symptoms. Both recovered after treatment with oseltamivir, with no additional cases reported. In July 2024, A fourth case was reported in Colorado, a dairy farm worker exposed to infected cattle. This individual developed conjunctivitis, was treated with oseltamivir, and recovered (UKHSA, 2024).
The CDC have since reported that there are a total of 57 confirmed cases of HPAI H5N1 to date as a result of the most recent US outbreak; 34 cases have been linked to exposure from cattle, 21 from poultry, and 2 from unknown sources (CDC, 2024b).
UKHSA reports of H5N1
A proportion of infections with H5N1 may be asymptomatic. Since 2023, the UK Health Security Agency has received 4 positive human detections of AI H5N1 clade 2.3.4.4b in exposed persons on farms in England where AI H5N1 was also confirmed in the poultry on site. 144 individuals from 8 infected premises have been tested, of which 4 were positive (2.7%). All detections were identified as part of an ongoing enhanced surveillance study of asymptomatic workers exposed to premises infected with AI. It remains unclear whether the detections in these asymptomatic individuals reflected deposition of viral particles on the mucosal surface (in other words, not causing infection) or infection with active viral replication (uncertainty) (UKHSA, 2023).
Global reports of H5N1
Symptomatic human cases of AI H5N1 continue to be detected globally, including cases of clade 2.3.4.4b. Since 2023, prior to the latest human cases in the USA associated with exposure to infected dairy cattle, detections in humans either confirmed to be, or associated with, clade 2.3.4.4b viruses have been reported in Ecuador, China and Chile. These cases were not fatal but did develop severe disease requiring hospitalisation (UKHSA, 2024).
Vulnerable groups
Vulnerable groups vary by strain for influenza. Limited information for B3.13 as is a newly emerging strain. Different groups will be vulnerable to different strains (uncertainty).
Risk characterisation
The probability that UK consumers will receive infectious exposures to influenza of avian origin via imported US milk and dairy products (e.g., pasteurised cheese, colostrum, yoghurt, ice cream) is assessed to be Negligible. This is a change from “very low” in the previous risk assessment. Several factors influenced this conclusion. These include the FDA regulation that all milk and dairy products that are exported to the UK must be pasteurised. In addition, the total volume of US dairy products exported to the UK is approximately 1,066,214 kg. Of this, only a small volume, specifically 8,493 kg, could be aged cheeses made from unpasteurised milk. There has also been no evidence to suggest oral infectivity of influenza virus in general for humans. The FSAs previous risk assessment concluded that the risk was Very Low as at the time it was not certain that dried milk powder imported from the US has undergone processing that would eliminate any infectious virus present. Since then, APHIS have confirmed that all exported dairy products are pasteurised.
The uncertainty in this estimate is Medium, primarily due to the effectiveness of pasteurisation in inactivating the virus. LTLT pasteurisation has been shown to reduce infectious virus in milk by more than 6 Log. HTST pasteurisation can also reduce infectious virus in milk between 4.44 and >6 Log. The majority of published studies that have assessed the effects of pasteurisation on H5N1 contaminated milk have concluded that it is highly effective in reducing the levels of virus (>6 Log). Two studies concluded that pasteurisation might be less effective at reducing levels of virus . “Medium” is defined as “There are some but no complete data available; evidence is provided in small number of references; authors report conclusions that vary from one another”. Furthermore, data has suggested that some cheese making processes, including ageing, can reduce the infectivity of the avian influenzas. However, the extent of H5N1 inactivation during cheese making is still uncertain.
The probability that UK consumers will receive infectious exposures to influenza of avian origin via imported US beef and offal is assessed to be Negligible, due to imported US beef accounts for a small proportion of beef consumed in the UK (0.0024%). Furthermore, the effectiveness of cooking to appropriate temperatures reduces infectious virus present. Testing of 208 muscle samples to date only detected virus in one animal at low levels. “Negligible” is defined as “So rare that it does not merit to be considered.”
The uncertainty in this estimate is Medium, due to the lack of surveillance data for infected beef herds and limited information on levels of the virus in beef. “Medium” is defined as “There are some but no complete data available; evidence is provided in a small number of references; authors report conclusions that vary from one another.” As the uncertainty is “Medium” and this is a rapidly evolving situation, any new evidence that may require this rapid risk assessment to be updated will be actively monitored.
Severity of infection
In the event of infection, the severity of detriment is assessed to be Low. Recent data from the CDC indicates there have been multiple confirmed human cases of HPAI (H5N1) linked to dairy cattle, with symptoms ranging from milk conjunctivitis to mild respiratory symptoms. As of November 2024, there have been 34 human B3.13 cases associated with cattle (CDC, 2024a), although these may be an underreporting due to mild infections. Most of the human cases were in farm workers; hence, the CDC suggests those who have job-related exposure to dairy cattle, are at a greater risk of contracting HPAI virus (CDC, 2024b). “Low” is defined as "Mild illness: not usually life-threatening, usually no sequelae, normally of short duration, symptoms are self-limiting (e.g., transient diarrhoea). This assumption that the currently circulating strain does not commonly result in severe infection is based on the observation that if someone is severely affected by an influenza-like illness in the US they would normally report to medical professionals and be hospitalised and tested. There is therefore some level of confidence that this strain is not resulting in large numbers of severe infections to date.
The uncertainty in this severity is Medium which reflects the recent emergence of this strain, the limited clinical data on infection with this strain so far, and the fact that reporting disincentives make it difficult to be certain that other human infections have not occurred. Additionally, foodborne exposure has not been confirmed and it is unknown whether severity of illness is any different compared to other routes of exposure e.g., inhalation. “Medium” uncertainty is defined as “There are some but no complete data available; evidence is provided in small number of references; authors report conclusions that vary from one another”.
This document specifically assesses the risk from the strain involved in the current outbreak in US dairy cattle (clade 2.3.4.4b genotype B3.13). Strains of influenza vary widely in their clinical presentation in infected humans. However, the majority of human cases of influenza of avian origin have been associated with Asian genotypes or clades. For influenza of avian origin as a whole (in this case, ingested via poultry products), the severity is considered to be High with Medium uncertainty (Kintz et al., 2023).
Key uncertainties
-
More evidence of the efficacy of pasteurisation, particularly HTST. Some reports of on the effectiveness of pasteurisation found less heat treatment was less effective; it is unclear why the variation in results has occurred. Some reports of the effectiveness of pasteurisation found heat treatment was less effective; it is unclear why the variation in results has occurred
-
Effects of aging on virus in cheese made from unpasteurised milk (by type)
-
Hazard characterisation includes information regarding similar strains due to lack of B3.13 information
-
Lack of surveillance for virus in US beef herds
-
Data on the distribution and levels of virus in muscle tissue of infected cattle
-
The severity of detriment is assessed as Low based on no evidence of severe illness and no vulnerable groups identified
-
There has also been no confirmation that severity of illness from foodborne exposure is any different compared to other routes of exposure such as inhalation
Abbreviations
Article updated on 21st August 2025.


.png)


.png)

