This is a joint FSA and FSS publication.
1. Introduction
In accordance with assimilated Regulation 2283/20152 on novel foods, the application RP2114 for the use of 6′-sialyllactose (6’-SL) sodium salt produced by derivative strains of Escherichia coli BL21 (DE3) for use as a novel food has been submitted for authorisation in each nation of Great Britain (GB).
Whilst it was a Member State of the EU, the UK accepted the risk assessments of the European Food Safety Authority (EFSA) in respect of authorisations for regulated food and feed products. Since the end of the transition period, the FSA and FSS have adopted equivalent technical guidance and quality assurance processes to be able to undertake GB risk assessments for regulated product applications.
To ensure our regulatory systems are risk proportionate, and resources are used effectively, the FSA and FSS have used the evidence submitted by the applicant and other information in the public domain, including the EFSA risk assessment opinion, to inform this safety assessment.
The FSA and FSS reviewers have evaluated the published EFSA risk assessment on the novel food and confirmed that this is appropriate for GB risk analysis. Consideration has been given to the processes undertaken to ensure the EFSA opinion is robust and whether there are any aspects that would require further review, such as specific issues for the countries of GB. The result of the assessment is that there is sufficient evidence of safety to conclude without requiring further risk assessment at this time.
This assessment represents the opinion of the FSA and FSS.
2. Details of other regulators’ opinions
6’-SL sodium salt produced by a genetically modified strain of E. coli K-12 DH1 has previously been authorised for use in Great Britain (FSA, 2022). 6′-sialyllactose (6’-SL) sodium salt produced by the genetically modified microorganism Escherichia coli NEO6 has also been assessed by the FSA and FSS (FSA, 2024b).
In 2022, EFSA published a risk assessment opinion (EFSA NDA Panel, 2022) on the safety of 6′-sialyllactose (6’-SL) sodium salt produced by derivative strains of Escherichia coli BL21 (DE3) for its use as a novel food. This opinion has been reviewed by FSA/FSS risk assessors.
Consideration was given to whether the novel food had been reviewed by other regulators. 6’-SL produced by this microorganism was notified to the FDA (Food and Drug Administration) in the USA through their Generally Regarded as Safe (GRAS) scheme and given the GRAS notice number GRN000922.
2.1. Methodology applied in the EFSA opinion
The EFSA assessment was conducted in accordance with the procedure as outlined in the EFSA scientific opinion 'Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283 (EFSA NDA Panel, 2016) and Commission Implementing Regulation (EU) 2017/2469.
2.1.1. Identity of the novel food
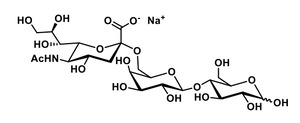
The novel food is 6’-sialyllactose sodium salt (6’-SL), a human-identical milk oligosaccharide in the form of a fine white to ivory powder, produced by fermentation from genetically modified E.coli BL21 (DE3). 6’-SL occurs naturally in human breast milk. The novel food is structurally equivalent to both 6’-SL found in human breast milk and commercially available 6’-SL. Other carbohydrates are present in small amounts including lactose (≤ 5.0 %) and 6’-sialyllactulose as outlined in Table 1.
6’-SL is characterised by:
IUPAC name:
N-acetyl α-neuraminic -(2→6)-β-D-Galactose-(1→4)-D-Glucose sodium saltCAS number: 157574-76-0
Molecular formula: C23H38NO19Na
Molecular mass: 655.53 g/mol
The identity of 6’-SL was determined by the following methods:
-
1D and 2D nuclear magnetic resonance (NMR) spectroscopy
-
Mass spectrometry
-
High-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD)
The FSA and FSS agree with EFSA that the identity of the novel food has been appropriately characterised.
2.1.2. Production process
The novel food is manufactured using a fermentation process using a genetically modified microorganism (GMM) derived from E. coli BL21 (DE3). Fermentation time is set to optimise product concentrations. The bacterial biomass is then separated from the culture broth. The separated product is isolated, purified and concentrated by a series of filtration, ion removal and decolourisation steps. Purified 6’-SL is then dried to a powder.
Information on the hazard identification, hazard characterisation, and exposure assessment for the genetically modified derivatives of E. coli BL21 (DE3) was provided in line with EFSA guidance (EFSA GMO Panel, 2011). The absence of endotoxins and Enterobacteriaceae is confirmed by immunological methods. The absence of BL21 (DE3) is confirmed by quantitative PCR.
The FSA and FSS agree that the production process is appropriately characterised, and any risks characterised and strategies for management outlined. No concerns were raised on this section of the assessment.
2.1.3. Compositional information and specification
The novel food is 6’-SL sodium salt produced by derivative strains of Escherichia coli BL21 (DE3).
Compositional data from five non-consecutive batches of 6’-SL confirms that the finished product consistently complies with the product specifications and that the production organism is removed.
The FSA and FSS agree that the compositional information has not highlighted areas of concern.
2.1.4. Stability
An ambient stability study was conducted on one batch of 6’-SL at 25°C and 25% humidity over 12 months. Additionally, an accelerated stability was conducted on one batch of 6’-SL at 40°C and 25% humidity over 3 months. No significant changes were detected in purity, appearance, odour, or solubility in either study.
As part of an ongoing storage study, further stability studies are being conducted for which data is available at 12 months. An ambient stability study conducted on two batches of 6’-SL at 25°C and 60% relative humidity. Additionally, an accelerated stability study at 40°C and 75% relative humidity was conducted on two batches of the novel food. In both studies, no significant changes were detected in 6′-SL sodium salt, carbohydrate, sodium and water content, physico-chemical parameters and water activity. There was no detectable microbial activity after 12 months. These results support a shelf life of 12 months.
The FSA and FFS agree with EFSA that the information on the production process, composition, stability and the specification of the novel food does not raise safety concerns.
2.1.5. History of use
6’-SL is a human milk oligosaccharide (HMO) that occurs naturally in breast milk and bovine milk. Thus, humans have been exposed to 6’- SL via consumption of human and bovine milk.
6’-SL sodium salt produced by a genetically modified strain of E. coli K-12 DH1 has previously been authorised for use in Great Britain (FSA, 2022). There are two market authorisations for 6’-SL sodium salt in the EU following positive EFSA opinions: 6’-SL produced by fermentation with E. coli k-1, and 6’-SL sodium salt produced by fermentation with E. coli BL21. 3’-SL has also received a positive EFSA opinion and been authorised for marketing in the EU.
The production strain E. coli BL21 (DE3) is already used in the production of food and pharmaceutical ingredients. BL21 (DE3) has previously been authorised for use in Great Britain in the production of the 3-fucosyllactose (3-FL) (FSA, 2024a). Previous EFSA authorisations for novel foods produced from E. coli BL21 (DE3) include lacto-N-tetraose (LNT). The FSA and FSS agree with EFSA’s assessment that no safety concerns arise from use of the production strain.
2.1.6. Proposed use and anticipated intake
The applicant has proposed that 6’-SL is to be used in food categories including infant and follow-on formula, foods for infants and young children, foods for special medical purposes and food supplements. The uses and use levels are outlined in Table 3.
6’-SL is largely indigestible and is fermented by the gastrointestinal (GI) tract microbiome. Therefore, the intended use of adding 6’-SL to infant and follow-on formula is to increase 6’-SL intake and to more accurately reflect the composition of human breast milk. The maximum daily intake from the proposed uses were 0.7 g for infants and young children and 1.8 g for those over 3 years of age.
The level of consumption from the novel food was compared to consumption of 6’-SL from breastmilk. Based on opinions by EFSA, (EFSA Panel on Nutrition, 2013; EFSA Scientific Committee, 2012), the applicant calculates an average daily intake of 6’-SL by breast fed infants of between 0.012-0.143 g/kg bw, by infants of 6.7 kg. The intended use level is similar to 6’-SL concentrations found in human milk.
The anticipated intake is calculated assuming that infants would be exclusively fed with the 6’-SL sodium salt-fortified formula for the first four months. Therefore, the novel food is not intended to be consumed if other sources of 6’-SL are consumed on the same day. This is detailed in Table 4.
Intake in mg/kg bw (body weight) per day is calculated using use levels (mg/day) and default body weights as defined in EFSA Scientific Committee (2012). The intake for each age group is calculated assuming 100% 6’-SL, whereas the novel food contains approximately 95% 6’-SL. Therefore, the figures presented in Table 4 represent an overestimation.
The high level of consumption of infant formula for infants aged 0-16 weeks results in exposure to the novel food of 260 mL/kg bw/day. Therefore, the high daily intake of 6’-SL sodium salt from infant formula containing 0.7 g/L 6’-SL sodium salt is 0.182 g/kg bw. The EFSA opinion notes that this level of intake from infant formula is higher than the estimated daily intake of 6’-SL of 0.133 g/kg bw in high consuming breast-fed infants of women with high mean concentration of 6’-SL. In their opinion, EFSA concluded that this was not a safety concern due to the wide range of intake levels of HMOs from human breast milk, and infants are exposed to similar intakes of HMOs. In a 2017 literature review, the mean concentration by publication of HMOs in human milk, excluding colostrum, was 5.341 g/L (Thurl et al., 2017). A more recent literature study found a maximum mean concentration of 8.40 g/L of 2’-FL in human milk (by secretor group, excluding colostrum) (Malih, 2024), which is higher than the value calculated here. The FSA and FSS further note that the intake of 6’-SL is unlikely to reach the estimated upper intake since the intake assessment assumes that the novel food is added at the maximum use levels to all the intended food categories consumed by infants. This estimate is not a true representation of the diet of infants.
The use level of 6’-SL sodium salt in foods for special medical purposes defined in Regulation (EU) No 609/2013 has to be assessed individually by the attending physician.
The FSA and FSS agree that exposure from the proposed uses is consistent with exposure to 6’-SL from human breastmilk.
2.1.7. Absorption, Distribution, Metabolism and Excretion (ADME)
Most human milk oligosaccharides (HMOs) are reported to undergo limited oral absorption. HMOs do not undergo significant digestion in the upper gastrointestinal tract but can undergo fermentation in the colon. HMOs are predominantly excreted unchanged in the faeces, with a small proportion excreted unchanged in the urine.
The absorption of 6’-SL from consumption of the novel food is not expected to differ from the intake of human milk oligosaccharides following infant consumption of breast milk. Therefore, this was not expected to pose a safety concern for any age groups including infants.
6’-SL is a highly indigestible HMO. In vivo studies in infants and rats have demonstrated that 1-2% of ingested HMOs are excreted unchanged in urine, with the remainder passing through the gastrointestinal tract and either being fermented by the resident microbiota or excreted unchanged in the faeces (Gnoth, 2000).
The ADME of HMOs are well understood and the information does not indicate any further areas of concern. The FSA and FSS agree that the ADME of 6’-SL is well characterised, and no areas of concern have been identified.
2.1.8. Nutritional information
The novel food is mainly composed of the oligosaccharide 6’-SL as a sodium salt that is structurally identical to naturally occurring 6’-SL which is found in human breast milk. HMOs are highly indigestible and are not considered to substantially contribute to the energy metabolism of infants (Gnoth, 2000). To calculate the energy contribution of 6’-SL, the applicant uses an EFSA opinion (EFSA, 2010) in which the energy conversion of dietary fibres, including indigestible oligosaccharides, was determined to be 2 kcal/g. Therefore, the energy contribution of 6’-SL at the proposed use level of 0.7 g is 1.4 kcal/L. This small increase is not expected to be nutritionally disadvantageous to consumers.
2.1.9. Toxicological information
2.1.9.1. Genotoxicity
The mutagenic potential of the HMO mix was assessed by in vitro bacterial reverse mutation tests (unpublished study LPT No.35504, Parschat, et al., 2020). The tests were performed on five S. Typhimurium strains (TA98, TA100, TA102, TA1535 and TA1537) exposed to concentrations up to 600 mg HMO mix per plate either in presence or absence of liver microsome fractions. No increase in revertant colony numbers was observed compared to control plates, demonstrating that the HMO mix is non-mutagenic up to 600 mg HMO mix/plate.
The in vitro mammalian cell micronucleus test (unpublished study, LPT No. 35907; Parschat, et al., 2020) demonstrated that the HMO mix is non-clastogenic and non-aneugenic (up to 2.6mg 6’-SL/ml) in the absence or presence of metabolic activation. The results from these in vitro studies support the conclusion that the novel food is not genotoxic.
The in vitro mammalian cell micronucleus test used concentrations of the HMO mix up to 60 mg/mL HMO mix tested with human peripheral blood lymphocytes in either presence or absence of metabolic activation. No statistically significant increase in micronucleus containing binucleate cells was found. There was no evidence of clastogenicity or aneugenicity in either presence or absence of metabolic activation up to 60 mg/mL HMO mix. The results from the in vitro studies support the conclusion that the novel food is not genotoxic.
The FSA and FSS agree that the evidence supports the conclusion that 6’-SL is not genotoxic.
2.1.9.2. Subchronic toxicity
EFSA reviewed three toxicological studies on a mixture of HMOs containing 6’-SL, 2’-FL (47.1%), 3-FL (16.0%), LNT (23.7%), 3’-SL sodium salt (4.1%), 6’-SL sodium salt (4.0%).
A repeated dose 90-day oral toxicity study in rodents (unpublished study, LPT No. 35907; Parschat et al., 2020) was conducted under GLP (OECD, 1998) according to OECD TG 408 guidelines (OECD, 2018). Each group consisted of 10 female and 10 male rats which were fed a standard diet or a standard plus 10% HMO mix ad libitum.
No deaths were reported, and no relevant clinical signs were observed. Episodes of decreased or increased food consumption were reported (in males only). A statistically significant reduction in spontaneous motility was reported in treated male rats without any change in any other functional observation test. A statistically significant increase in body temperature was reported for female rats. Variations in haematology (female only), clinical chemistry, urinalysis (in females only), organ weights, and histopathology (male only) were observed. The observed changes were of low magnitude and/or limited to one sex and were not considered to be biologically relevant.
In another 90-day repeat dose toxicity study where rats were given ad libitum a standard diet with or without 10% 6’-SL alone (unpublished study, LPT No. 36546), microscopic kidney deposits were observed in 5 out of 10 male rats and 2 out of 10 female rats. EFSA requested further information from the applicant to identify the nature of the microscopic kidney deposits. The composition of the deposits was demonstrated to be calcium phosphate. Administration of sodium salts is known to alter urinary composition and is associated with renal vascular changes in rats. Furthermore, when the batch of 6’-SL used in the study was reanalysed it was found to have a high sodium content (6.1%), which is outside the specifications of 4.2%. The current specification had not been set at the time of the study. EFSA concluded that the precipitate formation is a species-specific high dose effect and as such are not relevant to human health. 6’-SL sodium salt has previously assessed by the FSA and the contribution to sodium in the diet was found to be negligible (FSA, 2024b). This conclusion is consistent with previous EFSA assessments for 6’-SL from other microbial sources or other HMOs.
The FSA and FSS agree that sufficient toxicology data has been presented to support the application, and no areas of toxicological concern have been identified from the information reviewed.
2.1.9.3. Human studies
EFSA noted that a that a double-blind, controlled, randomised study was conducted in infants with infant formula containing a mixture of 5 HMOs mimicking the natural concentrations of the 5 most abundant HMOs (5.75 g/L total, comprising 52% 2′-fucosyllactose, 13% 3-fucosyllactose, 26% lacto-N-tetraose, 4% 3′-sialyllactose, and 5% 6′-sialyllactose) to determine tolerability, safety and effect on growth (Parschat, et al., 2021). The safety profile of the infant formula containing the HMO mixture was found to be similar to that of the infant formula alone. No concerns on growth or safety were reported. The FSA and FSS agree with the EFSA conclusion that this supportive of the safety of 6’-SL.
The FSA and FSS agree with the EFSA assessment of these studies that there are no significant results with relevance to human health.
2.1.10. Allergenicity
The allergenic potential of 6’-SL is negligible. The protein content in the novel food is low (≤ 0.01%). There are no reports of allergic reactions to human milk. E.coli BL21 (DE3) has a history of safe use in the production of food and pharmaceutical ingredients.
The potential for the genes used in the production strain of BL21 (DE3) to produce allergens was considered. The genes used in BL21 (DE3) are not derived from major allergens. Full-length FASTA alignments of amino acid sequences of the genes used to engineer E. coli BL21 (DE3) and version 19 of the AllergenOnline database showed that cross-reactivity with known allergens is not expected.
The FSA and FSS agree that the risk of allergic reactions is low under the proposed conditions of use.
3. Other regulators’ opinions and conclusions
EFSA found that the novel food is mainly composed of 6’-SL, identical to 6’-SL found in human milk. The novel food also contains small amounts of D-lactose, 6′-sialyllactulose, sialic acid, N-acetyl-D-glucosamine and related oligosaccharides, though this was not considered to impact the safety of the novel food.
The novel food is intended to be added to in infant and follow-on formula, foods for infants and young children, foods for special medical purposes and food supplements. EFSA noted that under some circumstances at very high use levels the estimated intakes per kilogram of body weight may exceed the high average natural intake. However, this was not considered to negatively affect the safety of the novel food under the proposed conditions of use due to the wide variations of 6’-SL concentrations in human milk, and the natural exposure levels in infants. On a body weight basis, the intake of 6-SL in infants is considered safe for other population groups as well. Human milk and other foods fortified with 6’-SL are not intended to be consumed on the same day; therefore, EFSA concluded that the novel food is safe under the proposed conditions of use.
4. Caveats and uncertainties
Anticipated use and exposure were calculated assuming that only the proposed food categories will be fortified with 6’-SL and that infants would be exclusively fed with the 6’-SL sodium salt-fortified formula for the first four months and would consume other sources of 6’-SL on the same day.
5. FSA-FSS conclusion for GB safety assessment
The application has been evaluated in line with ‘Guidance on the preparation and presentation of an application for authorisation of a novel food’ in the context of assimilated Regulation (EU) 2015/2283 (EFSA NDA Panel, 2016); presentation of an application for authorisation of a novel food in the context of assimilated Regulation (EU) 2015/2283 (EFSA NDA Panel, 2016); and assimilated Implementing Regulation (EU) 2017/2469, for purposes of the GB assessment.
The conclusions of the EFSA opinion (EFSA NDA Panel, 2022), which have been reviewed in detail by the FSA and FSS for the purposes of the GB assessment, are considered appropriate and consistent within the uncertainties and limitations identified by EFSA.
The FSA/FSS could not have completed the assessment of the product without the following data claimed as proprietary by the applicant:
-
Description of the GMM production organism;
-
Annexes to the application detailing testing to confirm the identity of the novel food;
-
Subchronic toxicology study - Parschat, et al., 2020 [unpublished];
-
Reverse mutation assay - Parschat, et al., 2020 [unpublished];
-
Micronucleus test - Parschat, et al., 2020 [unpublished];
-
Clinical study in humans - Parschat, et al., 2021 [unpublished].
6. Outcome of the assessment
The FSA and FSS has reviewed the applicant’s dossier, supporting documentation, and most notably the EFSA opinion (EFSA NDA Panel, 2022), and consider that there is sufficient evidence to conclude the safety assessment of 6’-SL without obtaining further information or conducting a further risk assessment.
The FSA and FSS conclude that 6’-SL is safe under the proposed conditions of use. The anticipated intake levels and the proposed use levels were not considered to be nutritionally disadvantageous. Risk managers may wish to consider precautionary labelling to ensure food and food supplements containing the novel food are not consumed on the same day.
In making this assessment, the FSA and FSS were able to rely on sufficient scientific evidence to make a conclusion on safety with no further questions to the applicant, and therefore no further risk assessment activities are necessary.
Sufficient evidence was available in the literature to give the FSA and FSS confidence about the safety of this novel food, for example, where other national food safety authorities had positively assessed the application using the same risk assessment guidance and core legal requirements which apply in GB.
The applicant provided sufficient relevant information as requested by the FSA and FSS.
The FSA and FSS review did not find any issues of divergence from the EFSA guidance (EFSA NDA Panel, 2016) or mutual approaches or new scientific issues for consideration.
There were no other specific issues that would require an assessment for the UK or the nations of the UK.