This is a joint FSA and FSS publication.
1. Introduction
The Applicant submitted an application to the Food Standards Agency (FSA) regarding the use of calcium tert-butylphosphonate as a nucleating agent in the manufacture of polyolefin (polypropylene and polyethylene) food contact materials (FCMs) and articles, including for use in contact with infant formula and human milk.
The utilisation of the additive as a nucleating agent in the manufacture of polyolefin materials brings a number of claimed advantages. The benefits described by the Applicant for polypropylene materials included reduced processing cycle time of injection moulded parts, increased stiffness and reduced hazing. For polyethylene materials, including rigid and film articles, the benefits described by the Applicant included reduced hazing, highly modified shrinkage properties, increased gloss and/or clarity, and/or reduction of water vapour and oxygen permeation rates.
The Food Contact Materials Joint Expert Group (FCMJEG) was requested to evaluate the information provided by the Applicant and provide a scientific opinion on the safety of calcium tert-butylphosphonate as an additive for use in the manufacture of plastic FCMs and articles.
2. Assessment
2.1. Identity and characterisation
The following information was provided on the substance:
-
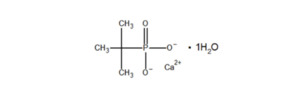
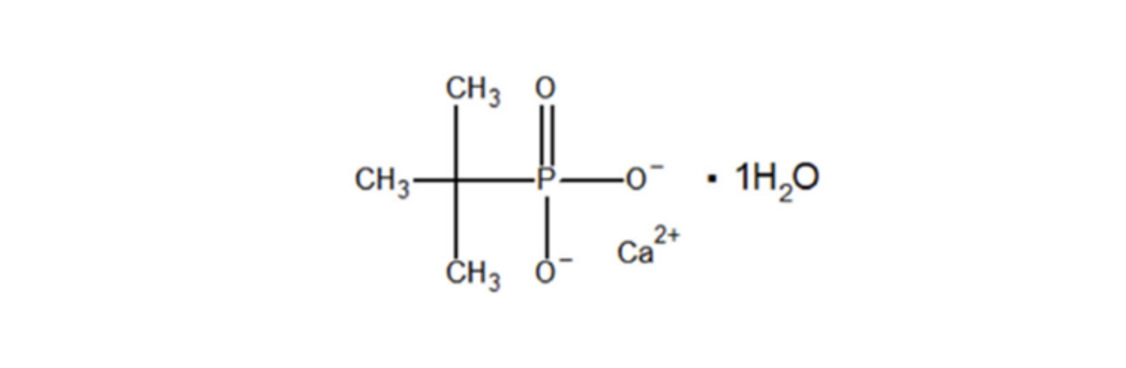
Chemical Name: Calcium tert-butylphosphonate
-
Synonym: Calcium t-butylphosphonate
-
CAS Registry Number: 81607-35-4
-
Molecular Formula: C4H11CaO4P
-
Structural Formula:
- Molecular Weight: 194.2 g/mol
2.2. Description and intended use
Members of the FCMJEG assessed the description and intended use of the product (in contact with all types of food (i.e. dry, aqueous, acidic, alcoholic, and fatty), including infant formula and human milk) and were satisfied with the additional information provided clarifying that the infant formula packaged in polyolefin-based food contact materials and articles containing calcium tert-butylphosphonate may be in both liquid (ready to be consumed as-is) or solid (intended to be reconstituted from a dry powder before consumption) state.
2.3. Spectroscopy data
The data (nuclear magnetic resonance (NMR) spectroscopy and attenuated total reflection infrared (ATR-IR) spectroscopy) provided by the Applicant to confirm the identity of the food contact substance was evaluated by the FCMJEG. They were content with the reasoning behind the selection of spectroscopic techniques and the data presented by the Applicant.
2.4. Manufacturing details
Calcium tert-butylphosphonate is made via a three-step manufacturing process. It has been agreed by the FSA to keep details of the raw materials used in the manufacturing process confidential.
The FCMJEG were satisfied with the manufacturing details provided by the Applicant.
The Applicant provided information on analysis of impurities present within the substance. The FCMJEG were satisfied with the information provided.
2.5. Purity
2.5.1. Specifications
Internal specifications for calcium tert-butylphosphonate, as sold, including purity and residual per cent water, plus known impurity concentrations, were submitted.
Overall, it was agreed that the information provided for specifications was satisfactory.
2.5.2. % Purity of the product
Calcium tert-butylphosphonate is manufactured to a minimum purity specification of 96%.
This specification was checked by gas chromatography (GC). Test results for % purity of three production batches were provided by the Applicant. The FCMJEG reviewed and accepted the methods and purity data; a triplicate percentage purity measurement was provided. An average purity of 99.6% was reported in the Certificate of Analysis (CoA).
2.6. Physical and chemical properties of substance
2.6.1. Melting point
Not applicable – calcium tert-butylphosphonate decomposes at high temperatures.
2.6.2. Boiling point
Not applicable – calcium tert-butylphosphonate decomposes at high temperatures.
2.6.3. Decomposition temperature
Calcium tert-butylphosphonate is expected to remain stable under the intended conditions of use.
The decomposition temperature of calcium tert-butylphosphonate is 468°C in air and 560°C in nitrogen (N2) atmosphere. One molecule of water of hydration is removed at 190°C.
2.6.4. Solubility
The solubility of calcium tert-butylphosphonate in water is approximately 0.411 g/L.
The Applicant confirmed that the solubility of calcium tert-butylphosphonate in water is very low but not zero. Any dissolved salt will consist of fully solvated Ca2+ cations and tert-butylphosphonate dianions. Its aqueous solubility was described by the Applicant. The determination was by reference to the concentration of phosphonate dianion in a saturated solution of calcium tert-butylphosphonate determined by liquid chromatography-mass spectrometry (LC/MS) in negative ionisation mode. External calibration was against standards of disodium tert-butylphosphonate, which is very soluble in water at room temperature. A stoichiometric correction from phosphonate dianion to calcium tert-butylphosphonate was made.
Calculations for determining the concentration of calcium tert-butylphosphonate from the chromatograms and calibration curve were provided by the Applicant.
The FCMJEG considered the analytical method used to determine the solubility of calcium tert-butylphosphonate in water. The Applicant provided additional information, which was found to be satisfactory.
2.6.5. Octanol/water partition (log P o/w)
The log Po/w of calcium tert-butylphosphonate is -2.5. Full analytical method parameters were provided.
The log Po/w provided by the Applicant would suggest that the compound is very water soluble, yet the Applicant goes on to state that its lack of aqueous solubility meant it would be unlikely to migrate. When requested to clarify whether the log Po/w was indeed -2.5, therefore indicating hydrophilicity the Applicant explained that when dehydrated, solubility of the compound decreases, thereby becoming hydrophobic. The FCMJEG accepted the applicant’s explanation.
2.6.6. Chemical properties
Information on the following chemical properties was provided:
-
Nature: Calcium tert-butylphosphonate is pH-neutral.
-
Reactivity: Calcium tert-butylphosphonate is an additive, which does not react with the plastic in which it is incorporated. On this basis, no reaction products are anticipated by the Applicant to be present in the substance, per se, or when calcium tert-butylphosphonate is used as intended. Furthermore, as this substance is not polymeric in nature, no oligomeric fraction would be present.
-
Stability: Calcium tert-butylphosphonate is stable under the intended conditions of use.
-
Hydrolysis: Calcium tert-butylphosphonate is not expected to hydrolyse under the intended conditions of use.
The Applicant stated that “phosphonate bonds are very stable chemically and will not be reactive under the conditions of food contact in polyolefins.”
It was also noted that “alkylphosphonic acids are typically 2-3 pKa units more acidic than the corresponding carboxylic acids. Thus, the metal salts from these acids cannot be dissociated by typical carboxylic acids in food simulants, i.e. acetic acid in water.” The Applicant cited Franz (2001) to support this assessment.
-
Intentional decomposition/transformation: Calcium tert-butylphosphonate is not intended to decompose or transform under the proposed conditions of use.
-
Unintentional decomposition/transformation: Calcium tert-butylphosphonate is not expected to unintentionally decompose or transform under the proposed conditions of use.
The Applicant stated that, in support of stability during use, a thermogravimetric analysis (TGA) of the substance had been provided in the original submission and the FCMJEG concurred with the conclusions.
- Interaction with food substances: Calcium tert-butylphosphonate is not expected to interact with food substances.
The Applicant stated that “The molecule is highly insoluble in most solvents, is stable chemically, and is not easily hydrolysed in the presence of food substances”.
2.7. Intended application of the substance
2.7.1. Food contact material
Calcium tert-butylphosphonate is intended to be used as a nucleating agent in the manufacture of polyolefin food contact materials and articles including polypropylene (PP), linear low-density polyethylene (LLDPE), low-density polyethylene (LDPE) and high-density polyethylene (HDPE), at a maximum level of 0.15 weight per cent of the polyolefin.
2.7.2. Technological function/maximum process temperature
The relevant supporting information for this section was submitted by the Applicant.
In PP, calcium tert-butylphosphonate reduces the processing cycle time of injection moulded parts, thereby increasing the stiffness of the part, and, to a greater degree, reducing the haze of the parts.
In PE, including both rigid and film articles, the primary effects of calcium tert-butylphosphonate are highly modified shrinkage properties (indicative of strong nucleation), reduction of haze, increased gloss and/or clarity, and/or reduction of water vapor and oxygen permeation rates. The most desirable functionality of calcium tert-butylphosphonate is demonstrated by the large effects on permeation rate. In HDPE blown film, calcium tert-butylphosphonate functions to significantly reduce the permeation rate of water vapour and oxygen.
Polyolefins that contain calcium tert-butylphosphonate will experience a maximum temperature of 190°C during extrusion and 230°C during injection moulding.
2.7.3. Maximum percentage in formulation
Calcium tert-butylphosphonate will be used at a maximum level of 0.15 weight per cent (1.5 mg/kg) in polyolefins.
2.7.4. Contact with food
Polyolefins containing calcium tert-butylphosphonate will be used in contact with all types of food (i.e. dry, aqueous, acidic, alcoholic, and fatty), including infant formula and human milk.
2.7.5. Contact time and temperature
Calcium tert-butylphosphonate is intended to be used in polyolefins in contact with food without limitations on temperature. Actual temperature contact conditions will be limited only by the physical and chemical properties of the finished polymer containing calcium tert-butylphosphonate at the target use level.
Polyolefins containing calcium tert-butylphosphonate are expected to contact foods at temperatures up to and including 130°C for short durations (e.g. < 15 minutes), such as microwave applications (excluding susceptor (i.e. microwaving with a metallic-film cover) applications), as well as longer durations (up to 2 hours) cooking and storage, where the polymer remains stable in contact with food at temperatures up to and including 100°C.
2.7.6. Migration
In migration studies, the conventional surface-to-volume ratio of 6 dm2/kg was used.
2.7.7. Treatment of food contact material prior to use
No specific treatment of polyolefins containing calcium tert-butylphosphonate (e.g. sterilisation, cleaning, rinsing, irradiation) is required prior to use in contact with food. The finished polyolefin should otherwise be technically suitable for the intended use in food contact applications.
2.8. Data on migration of substance
Relevant supporting information for this section was submitted by the Applicant. Additionally, information on the stability of the material with temperature, was provided.
2.8.1. Test sample
Calcium tert-butylphosphonate is intended for use as an additive in polyolefins, including PP, LDPE and HDPE.
The Applicant stated that “it is generally accepted that migration from LDPE represents a suitable worst-case surrogate for migration of an additive for other polyolefins discussed herein.”
A detailed justification as to why LDPE represents a worst-case surrogate was provided by the Applicant and was agreed by the FCMJEG to remain confidential.
Therefore, migration was measured using LDPE-based test plaques (LyondellBasell Petrothene NA217-000) containing the maximum intended use level (0.15 wt.%) of calcium tert-butylphosphonate.
Calcium tert-butyl phosphonate was added gravimetrically to the LDPE polymer (1.5 g of calcium tert-butyl phosphonate per 1,000 g of additive/polymer), mixed in a 10 L Henschel blender at high intensity and then compounded in a Deltaplast extruder at 190°C. Plaques were prepared using a 40-ton Arburg injection moulded with a barrel temperature of 230°C.
Physical properties of the test plaque were provided by the Applicant in Table 2.
The test plaques were immersed in food simulant with contact on both sides of the plaque. Migration can be considered from both sides of the plaque, and using two plaques, this gives a total surface area of 24 in2 (2 x 12 in2), or 154.8 cm2.
The test sample did not undergo any treatment step (e.g. cleaning or washing) prior to analysis.
2.8.2. Test food(s)/food simulant(s)
The Applicant stated that the following food simulating solvents were used in the migration tests:
-
Simulant A – Ethanol 10% (v/v)
-
Simulant B – 3% Acetic Acid
-
Simulant D2 – Ethanol 95% (v/v) (in lieu of vegetable oil/Food Simulant D2).
NOTE: The Plastics Regulation 10/2011, Annex V, Ch. 2.1.3 (as amended), states “If the testing conditions representative for the worst foreseeable conditions of intended use of the material or article, are not technically feasible in food simulant D2, migration tests shall be done using ethanol 95 % and isooctane.”
In response to a request to justify their selection of 95% ethanol over isooctane, the Applicant provided the following:
The Applicant commented that “Migration testing was conducted solely in 95% ethanol. The Plastics Regulation No. 10/2011 further states in this same Annex V, Section 2.1.3, that ‘[i]f it is found that carrying out the tests under the combination of contact conditions specified in Tables 1 and 2 causes physical or other changes in the test specimen, which do not occur under worst foreseeable conditions of use of the material or article under examination, the migration tests shall be carried out under the worst foreseeable conditions of use in which these physical or other changes do not take place.’”
It is well-established that isooctane is both known and expected to cause swelling in polyolefins, as further discussed in Feigenbaum et al., (2000). More specifically, during contact with a polymer and a food simulant, two mass transfer phenomena occur: sorption of the solvent by the polymer (also known as polymer swelling); and migration of chemical species from the polymer into the food simulant. Ideally, in migration tests, there would be minimal swelling of the polymer so that only migration of chemical species from the polymer can be measured. Swelling of the polymer, in contrast, is not reflective of actual use conditions, nor a representation of actual migration values.
For this reason and based on the documented potential incompatibility of vegetable oil and the well-established interaction between isooctane and polyolefins, 95% ethanol represents the “the worst foreseeable conditions of use in which these physical or other changes do not take place.” Therefore, 95% ethanol was chosen as the appropriate alternative food simulating solvent for food simulant D2, as specified in The Plastic Materials and Articles in Contact with Food (England) (No.2) Regulations 2006.
The FCMJEG queried why the Applicant had not conducted a migration test using food simulant E, 2,6-diphenyl-p-phenylene oxide (commonly known as Tenax®), when taking into consideration the Plastics Regulation 10/2011, Annex V, Ch. 2.1.3, which states “in addition a migration test shall be done using food simulant E if the temperature under the worst foreseeable conditions of intended use exceeds 100°C. The test that results in the highest specific migration shall be used to establish compliance with this Regulation.” The FCMJEG requested that the Applicant provide migration testing data conducted using 2,6-diphenyl-p-phenylene oxide or justification as to why a migration test into simulant E was not required.
The Applicant confirmed that “The use of different food simulants was to represent different types of foods. The food simulant 10% ethanol was used to mimic migration into aqueous foods, while 3% acetic acid was used to mimic acidic foods. The use of vegetable oil, 95% ethanol, and 2,6-diphenyl-p-phenylene oxide as food simulants was to mimic migration into fatty foods and would account for migration of lipophilic substances. Therefore, it was not expected for the substance to migrate at a higher degree in vegetable oil, 95% ethanol, and/or 2,6-diphenyl-p-phenylene oxide than in 10% ethanol or 3% acetic acid. As shown in the migration tests, calcium tert-butylphosphonate migrated at a higher level in 3% acetic acid than 95% ethanol and 10% ethanol. Thus, due to the nature of calcium tert-butylphosphonate, the substance was most soluble in 3% acetic acid as compared to the other food simulants (vegetable oil, 95% ethanol, and 2,6-diphenyl-p-phenylene oxide) and they expected migration to 3% acetic acid to result in the highest specific migration.”
Whilst the effects of isooctane on the physical properties of a polyolefin-based material were acknowledged, the FCMJEG noted that migration testing from polyolefins into isooctane had previously been reported in the scientific literature. Therefore, the FCMJEG requested that the Applicant provide a migration test in isooctane as per the method described and used by the Applicant with the other food simulants, or alternatively, to provide additional data/justification to support their decision not to conduct a migration test in this simulant.
The Applicant responded with the following: “We acknowledge that the migration testing can be conducted with isooctane. However, based on scientific literature, the use of isooctane with polyolefins would cause the polymer to swell, resulting in migration behavior that would be considered exaggerative. Migration testing is meant to mimic actual use conditions. Thus, testing migration from a polyolefin into isooctane would most likely result in exaggerative migration levels that would not reflect the actual use conditions of the substance and the migration behavior of the substance from polyolefins to food.”
For clarity, the Applicant was also requested to provide a more detailed explanation on how they concluded that the substance was more soluble in 95% ethanol than vegetable oil.
The Applicant responded with the following: "When a 1 ppm solution of the disodium tert-butylphosphonate in 95% ethanol is vortexed with same amount of vegetable oil, the SIR (Selected ion recording) chromatogram of the 95% ethanol layer shows a 9.4% increase in peak area. This indicates that other species in the vegetable oil can interfere with the m/z=139 signal obtained by SIR. The applicant considered the increase in signal is a clear indication that no disodium tert-butylphosphonate salt is lost to the vegetable oil after vortexing, so 95% ethanol was a much more suitable solvent for these migration studies. From a relative polarity standpoint, organic ionic salts are highly polar in nature, much like water and ethanol. Oils (like vegetable oil) are mixtures of polar and nonpolar fatty alcohols, esters, and triglycerides- which are quite hydrophobic in nature. Ionic salts are in general more hydrophilic in nature (as they are often made in water) therefore, would exhibit a higher solubility in water and alcohols compared to vegetable oils. "
FCMJEG Members did not agree with the conclusion that the increase in signal is a clear indication that no disodium tert-butylphosphonate salt is lost to vegetable oil after vortexing. However, FCMJEG members accepted the hydrophobic nature of the vegetable oil and were satisfied with the additional information provided by the Applicant.
The FCMJEG noted that the Applicant provided the concentration found in the migrants as the basis of the results for the migration tests. For clarity, the Applicant was requested to confirm the actual migration values and to update the relevant tables to also include the actual migration values.
The FCMJEG requested a summary of the migration test report results, which was provided by the Applicant as seen below in Tables 3-6.
The Applicant also provided the summary data for calcium tert-butylphosphonate in three spiked migration solutions (10% ethanol, 3% acetic acid and 95% ethanol). The average recovery percentages for calcium tert-butylphosphonate in spiked (0.015, 0,030, 0.060 µg/mL) 10% ethanol migration solutions were 81.9%, 99.6% and 95.9%, respectively. The average recovery percentages for calcium tert-butyl phosphonate in spiked (0.015, 0,030, 0.060 µg/mL) 3% acetic acid migration solutions were 80%, 87.1% and 93%, respectively. The average recovery percentages for calcium tert-butyl phosphonate in spiked (0.015, 0,030, 0.060 µg/mL) 95% ethanol migration solution were 93.3%, 98.1% and 93%, respectively.
As provided in the migration test report, migration of calcium tert-butylphosphonate was reported in terms of concentration in the migration solution, µg/mL (w/v ppm).
2.8.3. Contact mode
Both sides of the test plaque were fully exposed to food simulant (i.e. total immersion) and were included in the calculation of the contact area. Each migration test was performed using two plaques immersed in 250 mL of food simulant and prepared in triplicate.
2.8.4. Contact time and temperature
The Applicant provided a response to a query regarding their choice of running the migration test once despite the fact that they had applied for approval for repeated-use articles.
Migration testing was conducted for 10 days. The test was initially conducted at 100°C for 2 hours, followed by 40°C for 238 hours.
The Applicant indicated that the migration test was originally conducted in support of FDA FCN 2011 and represents a high temperature contact phase, followed by a long-term storage phase.
The Applicant also noted that “in accordance with the assimilated Plastics Regulation 10/2011, Annex V, 2.1.4, ‘for contact times above 30 days (long term) at room temperature and below, the specimen shall be tested in accelerated test conditions at elevated temperature for a maximum of 10 days at 60°C… [however], for storage at room temperature the testing conditions can be reduced to 10 days at 40°C if it is shown by scientific evidence that migration of the respective substance in the polymer has reached equilibration under this test condition.’”
Migration solution samples were taken at the 2-hour timepoint (i.e. after the high temperature phase [100°C]), then again at three time points (24 hours, 96 hours, and 240 hours) during the lower temperature, long-term storage phase (40°C).
Migration of the substance was measured in all food simulants, at all time points, and in each case was at the limit of detection (LOD). Because migration did not increase with time, migration can be considered to have reached an equilibrium. Therefore, migration testing at 100°C for 2 hours, followed by 40°C for 238 hours may properly be considered a representative worst case for measuring the migration of the substance when placed in contact with food under all foreseeable temperature conditions.
The Applicant was requested to supply evidence, which demonstrates that a material or article incorporating the food contact substance complies with The Plastics Regulation (EU) No.10/2011, Annex V, 2.1.6 which states that “if the material or article is intended to come into repeated contact with foods, the migration test(s) shall be carried out three times on a single sample using another portion of food simulant on each occasion”. The Applicant responded with the following: “The Plastics Regulation No. 10/2011 further states in this same Annex V, Section 2.1.6, that ‘if there is conclusive proof that the level of the migration does not increase in the second and third tests and if the migration limits are not exceeded on the first test, no further test is necessary.’”
2.8.5. Surface to volume ratio
For both migration studies, a surface-to-volume ratio of 24 in2/250 mL or 6 dm2/kg was used.
2.8.6. Migration testing protocol
Details of the migration testing protocol are confidential.
The migration of calcium tert-butylphosphonate in all food simulants was close to or below the limit of detection (up to 10 μg/kg). Overall, the FCMJEG were satisfied with the additional information provided for the test foods/food simulants.
2.8.7. Non-intentionally added substances (NIAS)
The FCMJEG requested further information on whether the applicant had conducted any additional testing to identify any NIAS and to provide scientific justification if any NIAS testing was not undertaken.
The applicant provided chromatograms of the blank and controls in their Migration Test Report to demonstrate that the identified peaks were not by-products of impurities of calcium tert-butylphosphonate; therefore, no identification or NIAS testing was attempted.
The FCMJEG agreed that no other substances are expected to occur from calcium tert-butylphosphonate due to its chemical properties.
2.9. Data on residual content of substance in the FCM
2.9.1. Actual content
Calcium tert-butylphosphonate does not react with LDPE, nor is it volatile; therefore the amount of additive incorporated into the polymer is the actual content expected to be present in the test materials. Because there is no expectation of loss of calcium tert-butylphosphonate, the presence of the additive at the intended use level in the actual test material used in migration experiments was not determined by analytical data.
2.10. Microbiological properties of the substance
This section is not applicable, as there are no microbiological considerations when calcium tert-butylphosphonate is used as intended. Calcium tert-butylphosphonate is neither intended nor expected to function as an antimicrobial.
2.11. Toxicological data
The toxicological data for calcium tert-butylphosphonate are presented below.
2.12. Genotoxicity
2.12.1. Bacterial reverse mutation assay (Ames test)
The study was conducted in accordance with test guideline OECD no.471.
Salmonella typhimurium strains TA1535, TA1537, TA98, and TA100 and Escherichia coli strain WP2 uvrA were exposed to calcium tert-butylphosphonate at concentrations ranging from 1.5 –5,000 µg/plate with and without metabolic activation. Dimethyl formamide was selected as the vehicle in both studies.
The first experiment used the plate incorporation method, and the second experiment used the preincubation method. Both assays were performed in the absence and presence of metabolic activation by phenobarbital/β-naphthoflavone-induced rat liver S9 fraction (S9-mix).
Six concentrations in the range of 15–5000 μg/plate were applied in the second experiment, while the test item was tested at eight concentrations ranging from 1.5 to 5,000 μg/plate in the first experiment. On triplicate plates, all concentrations were assessed along with positive and negative (vehicle) controls.
A test item precipitate (white and granular in appearance) was noted in both the presence and absence of the metabolic activation system (S9-mix) at 5,000 and from 1,500 μg/plate in Experiments 1 and 2, respectively.
Results for the negative controls (spontaneous mutation rates) and viability were acceptable, both with and without metabolic activation. All of the positive control chemicals used in the test induced marked increases in the frequency of revertant colonies, both with and without metabolic activation as appropriate. Thus, the sensitivity of the assay and the efficacy of the S9-mix were validated.
Under the conditions of this study, the test item did not induce an increase in the frequency of revertant colonies that met the criteria for a positive result, either with or without metabolic activation (S9-mix). Under the conditions of this test, the test item was considered to be non-mutagenic.
2.12.2. In vitro micronucleus test in human lymphocytes
The study was conducted in accordance with test guideline OECD No. 487.
Duplicate human peripheral blood lymphocytes were used in the in vitro micronucleus assay to evaluate calcium tert-butylphosphonate (purity 98.4%). The test was conducted in accordance with GLP guidelines. The methodology for the cytokinesis block micronucleus assay was used. Demecolcine, mitomycin C, and cyclophosphamide served as positive controls. The test item was suspended in dimethyl sulfoxide (DMSO) since it was insoluble in the culture medium. Liver S9 from rats induced by phenobarbital and β-naphthoflavone was applied to duplicate lymphocyte cultures from a healthy donor for 4 hours, either in the presence of cytochalasin B (CytB) for 24 hours after recovery, or for 24 hours without S9 and then for a further 24 hours with CytB. In every experiment, concentrations of 50, 100, and 200 μg/mL test item were used.
The concentration of 200 μg/mL was the lowest precipitating concentration in all three exposure groups and the maximum concentration selected for evaluation of micronuclei in the binucleate cells.
For the test item and the positive controls, micronuclei were scored in 2,000 binucleated cells per concentration (1,000 from each culture), and in 4,000 binucleated cells (from 4 cultures) for the vehicle controls. A toxicity index known as the Cytokinesis-Block Proliferation Index was calculated using 500 cells per culture. Under no experimental circumstance with the test item did treated cultures show any increase in binucleated cells containing micronuclei or dose-related toxicity as compared to vehicle controls.
The criteria for a negative result were therefore achieved in all three of the exposure groups.
Whilst evaluating the toxicological data supplied by the Applicant, the FCMJEG sought clarification as to why the solvent that was used differed between the Ames test and the in vitro micronucleus test (DMF vs DMSO). Further information on the solvent selection was submitted by the Applicant, which the FCMJEG considered sufficient.
It was concluded that the test item, calcium tert-butylphosphonate, did not induce any statistically significant increases in the frequency of binucleate cells with micronuclei in either the absence or presence of a metabolising system. Calcium tert-butylphosphonate treatment did not show marked cytotoxicity.
The results from all three exposure conditions in the main experiment indicated that the frequency of cells with micronuclei was within the normal range in negative controls and significantly increased in positive controls compared to the historical data.
The test item was therefore considered to be non-clastogenic and non-aneugenic to human lymphocytes in vitro.
2.12.3. Overall summary of genotoxic studies
The available toxicology data, therefore, showed calcium tert-butylphosphonate to be negative in the in vitro Ames test and in vitro micronucleus (MN) assay and therefore unlikely to be of concern for genotoxicity, especially given its low exposure in humans.
2.13. Other considerations
EFSA has published guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA, 2017). In this age group, infant formula or human milk may be the only source of nutrition, and health-based guidance values for the general population do not apply without further considerations. Organ development and the absorption and distribution rates of a substance in infants and adults may differ. Risk assessment should be on a case-by-case basis, depending on whether the substance is added intentionally to food and is systemically available.
The FCMJEG noted that calcium tert-butylphosphonate was not intentionally added to food; however, it is an organophosphate compound, and such compounds can cause neurotoxicity. If migration and absorption occur, the extent of any transport across the blood brain barrier is unknown, which then may or may not lead to potential neurotoxicity in infants. Although the potential for exposure is probably very low, contact with infant formula and infant milk could not be evaluated. The main concern is the lack of data in this potentially sensitive age group, and that infants <16 weeks are expected to be exclusively fed on breast milk and/or bottle-fed infant formula, with potential exposure to the FCM from bottles.
The FCMJEG noted that if the applicant wishes to submit further data to aid in the assessment of the <16-week infant group then an extension of use application would need to be submitted.
3. Conclusions of the FCMJEG
Satisfactory information regarding the identity of the substance, its physical and chemical properties, the intended application of the substance, data on migration of the substance and toxicological data were submitted.
Results from the overall and specific migration test demonstrated that the migration of calcium tert-butylphosphonate was close to or below the limit of detection (up to 10 μg/kg).
Owing to the low migration of calcium tert-butylphosphonate as an additive under the conditions of use specified in the application, only limited toxicology testing was required.
Overall, there is unlikely to be a genotoxicity risk to health from the use of calcium tert-butylphosphonate as an additive in the manufacture of plastic materials and articles intended to be in food contact with food.
A potential health risk to infants (<16 weeks old) via feeding bottles, based on neurotoxic effects, could not be assessed due to lack of available data for this age group.
Overall, the FCMJEG considered that the information and data provided was sufficient to conclude that there was no concern for the general population from the use of calcium tert-butylphosphonate as an additive for use in food contact materials and articles. Use in contact with infant formula and human milk was excluded due to the lack of neurotoxicity data and the potentially sensitive nature of the infant group (<16 weeks).
Calcium tert-butylphosphonate was therefore recommended for approval for use as an additive as outlined in the application and specified above.
Abbreviations
Glossary
Acknowledgements
With thanks to members of the FCMJEG during the course of the assessment who were: Dr Stuart Adams, Dr Emma Bradley, Dr Gill Clare, Dr Sibylle Ermler, Dr Natalia Falagán, Dr Jenny Odum and Dr Michael Walker.