1. Statement of purpose
Glycerol (E422) is used in the production of low calorie and sugar free slushed ice drinks, functioning as a humectant, preventing the liquid freezing solid. Despite FSA recommendations (FSA, 2023) that slushed ice drinks were unsuitable for children aged 4 or under, and that refills were unsuitable for children aged 10 or under, incidents were still being recorded. Therefore, we have conducted a consolidated rapid risk assessment incorporating additional data to assess the risk to children with below average body weights for their age range. This was commissioned to be made publicly available to share our findings on the risk of glycerol in these products more widely.
2. Background and Introduction
Slushed ice drinks can contain glycerol (E422) as a substitute for sugar to maintain their slushy texture. Although glycerol is found in some other foods, it is added at much lower quantities than in slushed ice drinks (FSA, 2023). It is thought that the Soft Drink Industry Levy (HM Revenue & Customs, 2016; HM Treasury, 2018) has resulted in increased levels of glycerol being used to replace the sugar that has been taken out. Despite glycerol being typically low in toxicity, there are concerns about its impact on young children when consumed in large amounts over a short period (FSS, 2023).
The FSA conducted a baseline risk assessment on glycerol in slushed drinks following an initial incident where a toddler consumed 3 x 350 ml servings (1.05L) of a slushed drink in a short period of time and presented to paediatrics in shock, hypoglycaemic and unresponsive. Testing identified a high blood lactate concentration and a high urine glycerol concentration. Data on glycerol concentrations from the incident and local authority (LA) testing, along with compositional data from industry stakeholders indicated that glycerol is present at a varying range of concentrations depending on the slushed product; higher levels are present in low sugar slushed ice drinks and lower levels in carbonated slushed ice drinks. Consequently, the assessment has been presented in concentration ranges rather than focussing on specific products.
Following the initial incident in 2023, the FSA published voluntary advice (FSA, 2023) against consumption of these drinks by children aged 4 years old or under. This recommendation was based on a toddler aged 1.5 to 3 years consuming a 350 ml (12fl oz cup; a single-serving volume sourced from industry) slushed ice drink containing 50,000 mg/L glycerol (the highest concentration in use by industry) and assumed an effect threshold of 1 g/kg bw (1000 mg/kg bw) as recommended by external expert advice. It was envisaged that should any side effects occur, these would be mild and self-limiting.
Reported Cases
There are relatively few data on the acute toxicity of glycerol, which is generally considered to be low. However, following the initial incident, a small number of case reports of illness following consumption of slushed ice drinks continued to be documented after the completion of the baseline risk assessment and publication of voluntary guidance. However, it should be noted that these occurred in children aged 4 or under, where the voluntary advice was not displayed or not followed.
Suspected illness in children from glycerol in slushed drinks was investigated by Brothwell et al (2025), who summarised in their report several case reports from the UK and Ireland occurring between 2009 and 2024.
Brothwell et al (2025) conducted a retrospective review of 21 children, aged 2 years to 6 years 9 months (3 children: <2 years 11 months, 10 children: 3–3 years 11 months, 6 children: 4–4 years 11 months, 2 children: ≥5 years, data provided by the author), who became acutely unwell after consuming slushed ice drinks, mimicking symptoms of inherited metabolic diseases (IMD). The review found that 93% of children became unwell within 60 minutes of consumption. The median age at presentation of illness was 3 years and 6 months, with symptoms including decreased consciousness (94%), hypoglycaemia (95%), lactic acidosis (94%), pseudohypertriglyceridaemia (89%), hypokalaemia (75%), and glyceroluria in all urine samples. Enzymatic and genetic testing showed no underlying IMD in 14 patients, and for the majority who avoided slushed drinks (95%) there was no reoccurrence. In the months that followed, the one patient that went on to consume another slushed drink became symptomatic within an hour, progressing to vomiting and drowsiness. Of the 21 cases described, two occurred in children aged 5 or over at the time of the first incident.
The authors concluded that slushed ice drinks with glycerol may cause glycerol intoxication in young children, mimicking disorders of gluconeogenesis and glycerol metabolism. They suggested that this message should be communicated with clinicians, parents and carers, and that public health bodies should advise against these drinks for children under 8 years. This advice was formulated based on their collection of new data, an assumed serving size of 500ml, and a precautionary approach given all cases were reported in children aged 7 or under. The authors noted that children’s weights varied significantly within age groups, therefore using a higher age threshold (like 8 years) ensured that children of lower bodyweights for their age would not exceed the pharmacological dose of 125mg/kg bw/hour. The advice formulated by the FSA in 2023 was however based on the limited available data at the time (a single case report in a toddler) and assumed the consumption of 350ml slushed drink, thought to be the largest of the standard UK cup sizes. As a result, the recommendations against slushed drinks for children 4 years and under was considered appropriate guidance.
The paper by Brothwell et al (2025) is the first documented case series of adverse effects in children arising from exposure to glycerol in slushed ice drinks. This is a very useful case series, but there are some limitations on its use in risk assessment. Firstly, the study reports 21 cases in the past 15 years, of which 20 cases occurred between 2018 and 2024, which is a low number on which to draw firm conclusions. Additionally, there is a lack of key data such as dose estimates, bodyweights, and consumption details which are not recorded for each case report. This makes it hard to determine whether true dose dependent toxicity was being seen in these children, or whether it was an idiosyncratic response presenting in susceptible children.
FSA toxicological risk assessors have noted that the adverse effects reported in these cases are more severe than those associated with the pharmaceutical use of glycerol.
Risk Assessment
A baseline risk assessment was produced in response to the first reported incident in a toddler and focused on two populations; toddlers (aged 1.5-3; mean bodyweight 14.6kg) and adults (aged 19-64; mean bodyweight 70kg). These age ranges were chosen as the lowest end of the age range (i.e. 1.5-3 years) and the highest end of the age range (19-64 years) and are typically chosen when carrying out risk assessments for incidents. This captures and represents the full spectrum and risk within the general population and can be refined as necessary. This risk assessment assumed the consumption of 1 x 350 ml serving of a slushed ice drink product (identified as being the largest of the standard UK cup sizes) containing glycerol at concentrations of 10,000, 15,000, 20,000, 25,000, 30,000, 35,000, 40,000, 45,000, 50,000, 55,000 and 60,000 mg/L. The volume of slushed ice drink product containing glycerol at these concentrations that would need to be consumed by toddlers and adults to reach an exposure of 125 mg/kg body weight (bw), the lowest pharmacological effect level calculated by the European Food Safety Authority (EFSA), has also been estimated.
Millions of slushed ice drinks are sold annually, with only a very small number of adverse effects being reported. Therefore, to take a proportionate risk management approach, less conservative assumptions on the effect threshold were used to develop the FSA voluntary guidance. The FSA’s voluntary guidance published in 2023 was based on available data at the time, of a single reported case of illness. Recommendations from an external expert suggested a more appropriate threshold of 1000 mg/kg bw, based on a study by Virno et al (1963). The FSA compared exposures from the initial baseline assessment to this pragmatic threshold of 1000mg/kg bw, resulting in exceedances only in children under 4 years old, assuming mean body weights. This was consistent with known incidents and formed the basis of the voluntary guidance (FSA, 2023).
This consolidated rapid risk assessment details the initial baseline risk assessment and provides an additional scenario analysis for susceptible toddlers (1.5 – 3 years), children (3-10 years) and adolescents (10-17 years) of a lower body weight than average for their age, based on the new case data described by Brothwell et al (2025). For the purpose of this risk assessment, where the term ‘children’ is used, this encompasses the complete age range from young toddlers, aged 1.5 years, through to older adolescents, aged 17 years, unless otherwise specified.
The consolidated risk assessment provides additional data regarding exposure to glycerol at 50,000 mg/L in children aged 1.5 to 17, specifically at the 5th, 15th, and 25th percentiles (lower body weights than average), to evaluate the impact of lower body weight on glycerol exposure. The concentration of 50,000 mg/L glycerol was selected for the updated risk assessment as it represents the maximum glycerol concentration for slushed ice drinks as of 2023, when the FSA published their voluntary guidance (FSA, 2023). In the consolidated risk assessment, the volume of slushed ice drink consumed remained at 350ml, equivalent to a 12fl oz cup, which is a single-serving volume and thought to be the largest of the standard cup sizes used in the UK.
3. Hazard identification
Glycerol (E 422) is authorised as a food additive in accordance with assimilated Regulation (EC) No. 1333/2008 on food additives. It is permitted for use at quantum satis, i.e. it must not be used at a level higher than is necessary to achieve the intended purpose, in flavoured drinks.
The use of glycerol as a food additive was reevaluated by the EFSA Panel on Food Additives and Nutrient Sources added to Food, in 2017 (EFSA, 2017; 2022). They concluded that glycerol has low acute toxicity and that local irritating effects of glycerol in the gastrointestinal (GI) tract reported in some studies were likely due to the hygroscopic and osmotic effects of glycerol (EFSA, 2017).
4. Hazard characterisation
The EFSA Panel concluded that there was no need for a numerical Acceptable Daily Intake (ADI) and there was no safety concern regarding the use of glycerol (E 422) as a food additive (EFSA, 2017).
Glycerol did not raise concern with respect to genotoxicity. Reproductive and prenatal developmental studies were too limited to conclude on reproductive toxicity, but no dose-related adverse effects were reported. None of the animal studies available identified adverse effects from glycerol treatment.
Observations in humans
The EFSA Panel reviewed several studies where glycerol was therapeutically administered to humans.
Glycerol has been used intravenously (IV) or orally to treat cerebral oedema and induce osmotic diuresis. Clinical studies show that intravenous doses up to 60,000 mg over 1–4 hours for up to a week, or several weeks (Frank et al., 1981), and bolus oral doses starting at 1,500 mg/kg body weight followed by 500–700 mg/kg every 3 hours (Cantore et al., 1964), or 500–1,000 mg/kg, have been effective (Wald & McLaurin, 1982).
The EFSA Panel determined that the therapeutic oral use of glycerol for patients with diseases affecting physiological functionality was not relevant for its safety assessment as a food additive. However, glycerol has been prescribed to reduce intraocular pressure in adult glaucoma patients and before intraocular surgery (Bartlett, 1991), with oral bolus doses for this indication ranging from 1,000 to 1,500 mg/kg body weight (as a 50% solution) with a daily total oral dose not higher than 120,000 mg (about 1,700 mg/kg bw)(Gilman, 1990). This therapeutic use was considered relevant for the safety assessment of glycerol (E 422) as a food additive.
The EFSA Panel also reviewed studies where glycerol was administered orally, as a bolus, to patients with ocular disease or healthy controls at doses ranging from 1,000 to 1,500 mg/kg bw (Buckell & Walsh, 1964; Consul & Kulsretha, 1965; Drance, 1964; Friedman et al., 1980; Kornblueth et al., 1966; Krupin et al., 1970). The observed side effects from oral therapies to patients with ocular disease or healthy controls were nausea, headache, and vomiting, or none at all.
Glycerol can be used in sport, with athletes using it for hyperhydration before exercise, or rehydration following exercise (Coombes & van Rosendal, 2013). While the suggested dose depends on individual body size, it is estimated that 1 – 1.1g/kg bw is standard for hyperhydration with doses of 1.2g/kg bw being associated with a maximum increase in total body water (Coombes & van Rosendal, 2013; Wagner, 1999). For rehydration, it is suggested that 1g/kg bw per 1.5L of fluid consumed post exercise can accelerate the restoration of plasma volume (Van Rosendal et al., 2010).
Van Rosendal et al (2010) reviewed 28 studies investigating glycerol use in hyperhydration and rehydration in sport. These 28 studies involved 238 adult subjects, some of whom were trained athletes, with oral doses ranging from 500 to 1,500 mg/kg body weight. Side effects were reported in a few cases, including nausea, diarrhoea, gastrointestinal distress (bloating), and light-headedness (Van Rosendal et al., 2010).
The EFSA Panel considered glycerol therapy in diabetic patients where repeated use of oral and intravenous glycerol in diabetic patients has led to hyperosmolar non-ketotic coma (e.g. Oakley & Ellis, 1976; Sear, 1976). EFSA noted Sear’s (1976) conclusions that this condition typically occurs in individuals with maturity onset diabetes or pre-diabetes, rather than in non-diabetic subjects.
The EFSA Panel reviewed the studies of Wald and McLaurin (1982) where patients were given oral bolus doses of glycerol (500–1,000 mg/kg body weight every 3–4 hours) to manage intracranial pressures (ICPs). Individual doses ranged from 4,000 to 70,000 mg (average 54,000mg), administered via a nasogastric tube as a 50% solution. Intravenous mannitol was used if ICPs did not change, or if a large volume of solution was aspirated from the stomach, followed by another trial of glycerol 4-24 hours later.
Data was reported for 6 patients. Maximum ICP reduction and serum glycerol concentration occurred 60–90 minutes after ingestion, returning to pre-treatment levels around 3 hours later, in most cases. Based on the dose and time period range in this study, the EFSA Panel estimated the therapeutic dose required to reduce ICP was 125–333 mg/kg bw/hour. The EFSA panel considered that a conservative estimate of the lowest oral bolus dose of glycerol required for pharmacological effects was 125 mg/kg bw/hour. The EFSA Panel considered this dose range would also be responsible for the side effects observed in some patients (nausea, headache and/or vomiting). EFSA further concluded that the acute bolus exposure to glycerol (E 422) through its use as a food additive should stay below doses at which pharmacological or side effects could occur, therefore for the purpose of this risk assessment, the EFSA Panel’s determined value of 125mg/kg bw/hour was used as the reference point for risk characterisation. The EFSA Panel noted that infants and toddlers can be exposed to this dose by drinking less than the volume of one can (330 ml) of a flavoured drink.
The EFSA Panel recommended modifying the EU specifications for E 422 and also suggested that more information on its uses, use levels, and analytical data should be made available (EFSA, 2017).
External expert advice
Even assuming under reporting due to the mild nature of the reported side effects, the baseline assessment did not seem to be consistent with what was known about the initial incident and the history of use of the products. Consequently, the FSA sought expert advice from a member of its scientific advisory committee, the Committee on Toxicity, who had the relevant expertise concerning EFSA’s 2017 conclusions on the lowest oral dose of glycerol required to produce a pharmacological effect, which was 125 mg/kg bw/hour; the lower end of a range identified as 125-333 mg/kg bw/hour.
On reviewing the study by Wald and McLaurin (1982), the study from which the pharmacological dose was derived (125mg/kg bw/hour), the expert concluded this value was overly conservative and highlighted several limitations. Firstly, the study was not a dose-finding study, and the method used to determine the protocol dose of 500mg to 1000mg/kg was unclear. Additionally, the study aimed to reduce intracranial pressure (ICP) to an outdated target of 300mmH2O (equivalent to 22mmHg), which is now considered the threshold for treatment of ICP rather than the typical physiological value of 136mmH2O (10mmHg) (Hawryluk et al., 2019). The response rate varied significantly between individuals, likely due to the technical difficulties and lack of reproducibility in measuring ICP. Furthermore, adverse events were not described in detail, making it unclear whether glycerol at the stated dose produced any clinically significant effects. Lastly, changes in serum osmolarity were not significant until after several days of repeated glycerol dosing. For these reasons, the expert decided that the study by Wald and McLaurin (1982) was not the most suitable choice.
From reviewing the database, the expert considered a threshold of 1000 mg/kg bw would have been more appropriate (Virno et al., 1963). This was based on reports where bolus doses of oral glycerol over a range of 1000 to 1,500mg/kg bw were used for ocular therapy or rehydration, and reported either no adverse effects or, headache, nausea/vomiting or mild gastrointestinal distress. It was suggested that if the threshold for the pharmacological effect of glycerol was as low as EFSA (2017) suggested, there would likely have been more case reports of toxicity and significant consumer avoidance of glycerol-containing products. At the time of this assessment, severe glycerol toxicity from ingestion of slushed drinks had only been reported in one case, whereas the drinks continued to be consumed very widely, indicating that the drinks had a wide margin of safety in practice. It was concluded that a threshold based an oral dose of 1000 mg/kg appeared to be protective.
In summary, the baseline risk assessment suggested that slushed ice drinks would be unsuitable for most children, with those aged 16 years and below exceeding EFSA’s proposed threshold dose range of 125 – 133mg/kg bw/hour (see Table 3). At a glycerol concentration of 60,000 mg/L children aged under 6 years exceeded both EFSA’s range, and the pragmatic threshold based on an oral dose of 1000mg/kg bw as identified above, while at a glycerol concentration of 50,000 mg/L children aged under 4 years exceeded both thresholds; this was used to develop the FSA voluntary guidance.
It was subsequently noted by the external review that the sample sizes across the human observational studies were small, highlighting that if a reaction to glycerol was a rare complication, then adverse effects may not be evident in such small sample sizes. Further limitations were also described, specifically the lack of relevance of the age ranges within the studies (adults) compared to age ranges of the emerging incidents (children), and the lack of comparability between bolus IV administration of glycerol compared to consumption of glycerol in the form of slushed ice drinks.
5. Exposure assessment
Following the initial incident in a toddler, the product being assessed was assumed to be a slushed ice drink being consumed by a toddler (1.5 – 3 years) or an adult (19 – 64 years). The baseline exposure assessment was carried out in 2 parts. The first assumed that a toddler or adult consumed 1 serving (350 ml; the standard UK cup) of slushed ice drink in a short period of time. Exposure estimates for glycerol at concentrations ranging from 10,000 – 60,000 mg/L in slushed ice drinks are presented in Table 1. For these calculations, the average (mean) bodyweights for toddlers and adults were determined using data from years 1-11 of the National Diet and Nutrition Survey (NDNS), and the Diet and Nutrition Survey of Infants and Young Children (DNSIYC) 2011 (Bates et al., 2014, 2016; Department of Health, 2013; Roberts et al., 2018).
The second part of the baseline exposure assessment used the pharmacological threshold value of 125 mg/kg bw/hour and calculated the volume of slushed ice drink that would need to be consumed to reach an exposure of 125 mg/kg bw, by a toddler (1.5 – 3 years) or adult (19 – 64 years) at average (mean) bodyweight for their age, for each glycerol concentration. This indicated the level of consumption at which adverse health effects could start to occur and is presented in Table 2.
To ensure the voluntary FSA guidance published in 2023 was protective of the appropriate age range, as part of the baseline risk assessment exposures were calculated in children aged 1.5 to 17, at mean bodyweights, and compared to both EFSA’s threshold of 125 – 133 mg/kg bw/hour and the pragmatic threshold of 1000mg/kg bw. This is presented in Table 3. As previously noted, for the purpose of this risk assessment, the term ‘children’ is used to encompass the full age range of 1.5 to 17 years, unless otherwise specified.
Due to the occurrence of incidents which followed the publication of the guidance, plus the publication of the case series by Brothwell et al (2025), calculations were undertaken to assess the exposure of glycerol in children, with lower bodyweights for their age who might be more susceptible. In this expanded assessment, exposure to glycerol at 50,000mg/L (maximum level in use by industry) was estimated for children aged 1.5 to 17 years based on the 5th, 15th and 25th percentile bodyweights, to see how lower bodyweights in each age group would affect estimates of exposure. These are presented in Tables 4, 5 and 6.
Calculation Breakdown
The volumes in Tables 2, 4, 5 and 6 were calculated as follows:
350 (ml) / exposure amount (mg/kg bw) x 125 (mg/kg/bw) =
Example:
350 (ml) /1,800 (mg/kg bw) x 125 (mg/kg bw) = 24ml
For the example provided, this means that toddlers 1.5-1.9 years of age will potentially experience undesirable adverse health effects following consumption of 24ml.
This exposure assessment shows the varying estimated exposures to glycerol at concentrations ranging from 10,000mg/L to 60,000mg/L in toddlers (1.5 - 3 years; mean bodyweight of 14.6kg) and adults (19 – 64 years; mean bodyweight of 70kg). Exposures in children at lower bodyweights (1.5 to 17 years; 5th, 15th and 25th percentile) were estimated at 50,000mg/L only.
At mean bodyweights (Table 1), glycerol exposures in toddlers range from 240 mg/kg to 1400 mg/kg at concentrations of 10,000 mg/L and 60,000 mg/L, respectively. In contrast, adults experience significantly lower exposures, ranging from 50 mg/kg to 300 mg/kg across the same concentration range. Among children (Table 3), exposures at 20,000 mg/L vary from 105 mg/kg bw in older adolescents (16–16.9 years) to 583 mg/kg bw in younger toddlers (1.5–1.9 years). These values rise substantially at 60,000 mg/L, reaching 313 mg/kg bw in older adolescents and up to 1721 mg/kg bw in the youngest age group.
When considering lower than average bodyweights in children (i.e. the 5th, 25th and 15th percentile) at 50,000mg/L, children at the 5th percentile have the highest estimated glycerol exposures ranging from 360 mg/kg in older adolescents (16 - 16.9 years) to 1800 mg/kg in young toddlers (1.5 to 1.9 years). At the 15th percentile, exposures are slightly lower, ranging from 330 mg/kg to 1600 mg/kg and by the 25th percentile, exposures further decrease to a range of 310 mg/kg to 1500 mg/kg. The trend in the exposure data shows that as bodyweight increases, the exposure to glycerol per-kilogram decreases, meaning slightly more volume of slushed ice drink would need to be consumed to reach the same pharmacological effect.
6. Risk characterisation
For the purposes of this risk assessment, the pharmacological dose of 125mg/kg bw/hour will be used as a reference point for risk characterisation. This dose represents what the EFSA Panel considered to be a conservative estimate of the lowest oral bolus dose of glycerol required for a pharmacological effect. In addition, the pragmatic threshold based on an oral dose of 1000 mg/kg bw has also been considered.
In the baseline risk assessment, exposures were calculated for toddlers, and it was estimated that consuming 1 x 350 ml of slushed ice drink containing glycerol levels ranging from 10,000 - 60,000 mg/L would equate to glycerol exposure ranging from 240 to 1400 mg/kg bw (Table 1). Estimates for adults indicate that consuming 1 x 350 ml serving of slushed ice drink containing glycerol levels ranging from 10,000 - 60,000 mg/L would result in estimated exposures that range from 50 to 300 mg/kg bw (Table 1).
The EFSA Panel estimated that the lowest oral bolus dose of glycerol required for pharmacological effects or side effects was 125 mg/kg bw/hour. This was determined by taking the conservative lower dose from the estimated therapeutic dose required to reduce ICP of 125–333 mg/kg bw/hour. Based on the minimum and maximum glycerol concentrations of 10,000 and 60,000 mg/L in a slushed drink product and assuming that the 350 ml is consumed in an hour by a toddler weighing 14.6 kg, the exposure ranged from 240 to 1400 mg/kg bw. The maximum is approximately 11.5-fold that required for a pharmacological effect. This exposure could lead to adverse health effects such as nausea, headache and/or vomiting. The greater the exceedance of 125 mg/kg bw/hour, the more severe the adverse effects are likely to be.
Using the glycerol concentrations provided by stakeholders, it is possible to calculate the volume of these drinks that would need to be consumed in an hour to reach an exposure equivalent to the pharmacological effect level (125 mg/kg bw/hour). For the highest and lowest concentrations, the calculations show that:
-
A toddler could consume 30 ml of a slushed ice drink containing 60,000 mg/L glycerol or up to 180 ml of a slushed ice drink containing 10,000 mg/L, in an hour and would have an exposure at or below the exposure associated with causing pharmacological effects. Both volumes are significantly lower than the standard cup size of 350 ml.
-
An adult could consume up to 146 ml of a slushed ice drink containing 60,000 mg/L glycerol, or up to 880 ml of a slushed ice drink with a glycerol concentration of 10,000 mg/L in an hour and their exposure would be at or below that associated with pharmacological effects. This represents less than half of the standard cup size at the highest concentration and around 2.5 standard cups at the lowest.
As a result of the outcomes of the baseline risk assessment, the FSA developed voluntary guidance (FSA, 2023) assuming a maximum glycerol concentration at 50,000 mg/L. Where higher levels of glycerol were being used, they were reformulated down to this limit.
Risk managers used the estimates from the exposure assessment (Tables 1 – 2) and compared these estimates to the EFSA conservative threshold of 125mg/kg bw/hour and a more pragmatic threshold of 1000 mg/kg bw (Table 3), as suggested by an expert member from the Committee on Toxicity. This exposure assessment (Table 3) showed that children aged 1.5 to 3.9 (mean bodyweights) would exceed both thresholds when consuming 350ml slushed drink at 50,000mg/L.
Using the initial assumption of a maximum glycerol concentration of 60,000 mg/L, it was found that any child under 16 years old would exceed the EFSA conservative threshold. Similarly, with the highest concentration currently in use (50,000 mg/L, as advised by industry), any child under 13 years old would exceed the EFSA threshold. When comparing exposures to the pragmatic threshold of 1000 mg/kg bw, only children under 4 years old would exceed it. This was consistent with known incidents and formed the basis of the voluntary guidelines, which included a restriction on refills for children under 10 years old. The assumption was that if a child was particularly sensitive, or consumed a larger volume, any side effects experienced would be mild.
After implementation of this voluntary guidance, additional cases occurred in children aged 4 or below when the guidance was either not displayed or was not followed. Brothwell et al (2025) reported a median age of 3 years and 6 months from the 21 cases they analysed. It should be noted that only some of the cases reported by Brothwell et al (2025), have been formally reported to the FSA as incidents.
Following these additional cases plus the publication of additional case reports by Brothwell et al (2025), the exposure assessment was refined to assess the impact of assuming lower bodyweights on the estimated exposure to glycerol in children only, aged 1.5 to 17 years. The 5th, 15th and 25th percentile bodyweights were estimated using data from the NDNS, and exposure was calculated at a glycerol concentration of 50,000mg/L only, which is the maximum level currently used by industry.
At the 5th percentile bodyweight, exposures range from 360mg/kg bw to 1800mg/kg bw for children between 16-16.9 years and 1.5 to 1.9 years, respectively (Table 4). The lowest volume of slushed ice drink required to exceed the threshold of 125 mg/kg bw/hour, is 24ml for children aged 1.5 to 1.9 years, increasing to 120ml in children aged 16 to 16.9 years. In toddlers (1.5 -1.9, and 2 - 2.9) at the 5th percentile bodyweight, the volume of slushed drink that would reach the threshold of 125mg/kg bw/hour therefore reduces from 37ml (Table 2) to an average of 27ml (Table 4), when compared to the mean bodyweight.
At the 15th percentile bodyweight, exposures range from 330mg/kg bw to 1600mg/kg bw for children between 16-16.9 years and 1.5 to 1.9 years, respectively (Table 5). At this percentile, the volume of slushed ice drink required to reach the level of pharmacological effect ranges from 27ml (1.5 to 1.9 years) to 130ml (16 – 16.9 years). In toddlers (1.5 -1.9, and 2 - 2.9) at the 15th percentile bodyweight, the volume of slushed drink that would reach the threshold of 125mg/kg bw/hour reduces from 37ml (Table 2) to an average of 29ml (Table 5), when compared to the mean bodyweight.
At the 25th percentile bodyweight, exposures range from 310mg/kg bw to 1,500mg/kg bw in children between 16 – 16.9 and 1.5 to 1.9 years, respectively (Table 6). EFSA’s suggested threshold of 125mg/kg bw/hour would therefore be reached at a volume of 29ml (1.5 to 1.9 years), ranging up to 140ml (16 – 16.9 years). In toddlers (1.5 -1.9, and 2 - 2.9) at the 25th percentile bodyweight, the volume of slushed ice drink that would reach the threshold of 125mg/kg bw/hour reduces from 37ml (Table 2) to an average of 30ml (Table 6), when compared to the mean bodyweight.
In conclusion, at the 5th, 15th and 25th percentile bodyweight, children in all age groups (1.5 – 16.9) would exceed the threshold of 125mg/kg bw/hour by consuming the standard portion of 1 x 350ml slushed ice drink which could lead to health effects such as nausea, headache and/or vomiting. These effects would be more likely in children of a younger age, with a lower bodyweight than average for the UK population.
Using the same approach taken when developing the voluntary guidance, that is, consumption of a 350 ml cup and using the pragmatic threshold of 1000 mg/kg bw, children with bodyweights at the 5th percentile would exceed the threshold aged 6 years of age or below, while children at the 15th or 25th percentile at 5 years of age or below.
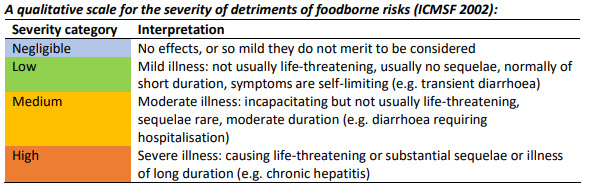
To present this risk assessment in a qualitative form, the scales for the frequency of occurrence and severity of foodborne risks and level of associated uncertainty that is described in the multidimensional risk assessment framework outlined by the Advisory Committee on the Microbiological Safety of Food (ACMSF, 2020) were used.
These are described in Figures 1, 2 and 3.
Exposure to glycerol from slushed ice drinks is known to cause glycerol intoxication in children, particularly those with lower bodyweights for their age. After the publication of the FSA’s voluntary guidance in 2023, cases continued to emerge with children experiencing adverse health effects from consuming glycerol in slushed drinks, as documented by Brothwell et al, who summarised 21 case reports in the UK and Ireland between 2009 and 2024. It is possible that there may be more cases of glycerol intoxication that are not documented due to under reporting and idiosyncratic reactions to glycerol cannot be excluded at any age.
Therefore, we consider the frequency of adverse reactions in the general population to be very low, (i.e. very rare but cannot be excluded).
As a result of some cases leading to hospitalisation in children, we consider the severity of illness from exposure to glycerol in slushed ice drinks to be medium (i.e. moderate illness, incapacitating but not usually life-threatening and of moderate duration). There could be a need for this qualitative assessment to be reconsidered if and when any new reports are submitted. This assessment is based on short term exposures. Currently there has been no reports of long-term effects associated with exposures to glycerol in slushed ice drinks. The outcome of this review should reduce exposures in the more susceptible individuals thus reducing any potential long term health issues
Due to the lack of complete data, and small number of references (Brothwell et al., 2025) we consider the level of uncertainty to be medium (i.e. there are some but no complete data available).
Uncertainties
There are a number of uncertainties to be considered as part of this risk assessment:
-
It is unclear what is in each formulation of slushed ice drink. Slushed ice drinks vary in their glycerol content, and there is no standardised recipe among food businesses, making it unclear without testing what concentration of glycerol is within final products.
-
It is unclear how accurately slushed ice drinks are being formulated for consumption within drinks machines, Manufacturers will supply instructions, but these need to be followed by venues supplying the drink.
-
This risk assessment assumes that a person will consume 350ml slushed ice drink in an hour, however it is often unclear how much slushed ice drink has been consumed in case reports. This means exposure may be under or overestimated; especially noting that certain establishments may offer free refills or serve in a way where a larger volume could be consumed, e.g. jugs for sharing.
-
The assumption of a 350ml serving size of slushed ice drink is based upon the UK maximum cup size of 12fl oz thought to be representative of a standard portion. It is possible slushed ice drinks may be served in jugs, or non-standard cups, which may exceed this volume, and would result in increased exposures to glycerol.
-
There are few data available on the oral toxicity of glycerol, and where cases of glycerol intoxication are reported causality may not be fully determined. As a result, there may also be under reporting of symptoms, where consumers are unaware that symptoms (e.g. nausea, headache) are related to the consumption of slushed ice drinks, which could lead to under reporting and underestimation of risk (BfR, 2025).
-
The more severe side effects associated with slushed ice drinks which are described in the case reports (hypoglycaemia, reduced consciousness and lactic acidosis) may occur in a variety of childhood illnesses, which may have led to under-recognition and under-reporting. Reports may have increased recently due to glycerol intoxication becoming more high profile.
-
The case reports indicate a more severe health effect than the side effects reported from pharmacological use; the reason for this remains unclear. This could be due to factors such as increased sensitivity in children (or a subset of children), the presence of sugar in earlier formulations mitigating the effect of glycerol, fasting, dehydration, or energetic activity taking place. It is also unknown whether, or how many, children aged 4 or under are consuming slushed iced drinks without adverse effects occurring.
-
The speed at which glycerol is consumed is important, however there is a lack of data on the rate at which slushed ice drinks are consumed children. The initial case involved consumption of a large quantity (~1L) over a short period of time, and the side effects arising from the pharmaceutical use of glycerol involve bolus dosing. In addition, it should be noted that the rate at which children consume glycerol slushed drinks may be significantly higher in those undertaking exercise than children at rest. However, there are few data on glucose metabolism, speed of consumption of drinks, or absorption of glycerol during exercise in children. Due to this lack of data, it is unclear how rate of consumption and exercise induced dehydration may alter the absorption and toxicological effects of glycerol.
-
Expert advice highlighted the possibility that adverse health effects resulting from glycerol in slushed ice drinks may be the result of idiosyncratic reactions within the population i.e. an unusual or abnormal reaction to glycerol that is specific to an individual. This means it would be difficult to identify a concentration of glycerol responsible for adverse health effects in these individuals.
-
The overall database is limited. There are very few data on the quantity of drinks consumed, and there is a lack of patient information within the case reports which do not include the body weights, family history and medical history of each child.
7. Conclusions
The re-evaluation by EFSA concluded that there was no need for a numerical ADI and no safety concern regarding the use of glycerol (E 422) as a food additive. The EFSA panel considered that a conservative estimate of the lowest oral bolus dose of glycerol required for a pharmacological effect to occur was 125 mg/kg bw/hour, this was a dose where side effects such as nausea, headache and/or vomiting could start to occur in some individuals. EFSA further concluded that the acute bolus exposure to glycerol when used as a food additive should stay below doses at which pharmacological or side effects could occur.
We have produced a consolidated risk assessment encompassing the baseline exposure assessment (Tables 1-3), and an additional exposure assessment for children of lower bodyweights for their age range (Tables 4-6). In the baseline exposure assessment, exposure to glycerol was calculated for toddlers and adults from consumption of 1 x 350 ml drink containing glycerol levels ranging from 10,000 – 60,000 mg/L (Table 1). In toddlers, these exposures resulted in a 2- to 11.5-fold range of exceedance of the pharmacological effect level of 125 mg/kg bw/hour, respectively. In adults, it was found that the exposures resulted in a 1- to 2.4-fold range of exceedance of 125 mg/kg bw/hour from consumption of 1 x 350 ml drink containing glycerol levels ranging from 25,000 – 60,000 mg/L. The adult exposures from 1 x 350 ml drink containing glycerol levels ranging from 10,000 – 20,000 mg/L were below 125 mg/kg bw/hour.
The volumes of slushed ice drinks that could be consumed and result in an exposure at or below that associated with pharmacological and side effects were also calculated (Table 2). For exposures to be below 125 mg/kg bw/hour, a toddler of average bodyweight could consume a maximum of 30 ml of a slushed ice drink containing 60,000 mg/L, or up to 180 mL of a product containing 10,000 mg/L glycerol. An adult of average bodyweight could consume up to 880 ml of a slushed ice drink product containing 10,000 mg/L or up to 146 mL of a product containing glycerol at a concentration of 60,000 mg/L in an hour. As a result of recent reported incidents, we were asked for a consolidated exposure assessment in younger children to capture exposure at lower bodyweights. As a result, the 5th, 15th and 25th percentile bodyweights were used to investigate the effects of lower bodyweights on exposure to glycerol in slushed drinks.
In the consolidated exposure assessment (Tables 4-6), it was found that at the 5th, 15th and 25th percentile bodyweight, children in all age groups (1.5 – 16.9) would exceed the threshold of 125mg/kg bw/hour by consuming the standard portion of 1 x 350mL slushed ice drink which could lead to health effects of nausea, headache and/or vomiting. These effects would be more likely in children of a younger age, with a lower bodyweight.
Using the 5th percentile bodyweight exposures as a worst-case scenario (Table 4), a toddler (1.5- 1.9 years) would only have to consume 24ml of slushed drink for an effect to occur, which is 14-fold less than the average consumption of slushed ice drinks at a standard portion of 350ml.
Both the new case data and the incidents reported to the FSA have largely occurred in children aged under 4, however, the effects are more severe than those reported from pharmaceutical use.
In conclusion, children with a lower bodyweight for their age may be more susceptible to glycerol intoxication leading to adverse health effects due to the high exceedance of both the pharmacological dose of 125mg/kg bw/hour, and the pragmatic threshold based on an oral dose of 1000mg/kg bw which underpins the current FSA voluntary guidance. While the FSA advice from 2023 which stated that slushed drinks are not recommended for children aged 4 or under was correct, the additional case reports and the potential severity of the effect suggests that a more conservative assumption on body weight would be appropriate, resulting in an a recommendation to update to the FSA voluntary guidance to the effect that slushed ice drinks are unsuitable for children aged 6 or under, this was based on the estimated exposures and taking into account the age range of the reported cases. However, as the mechanism underpinning the adverse effects is not fully understood the potential for an idiosyncratic reaction at any age cannot be excluded.
The advice that refills of slushed ice drinks are unsuitable for children aged 10 or under remains unchanged.
Abbreviations
ADI: Acceptable Daily Intake
FSA: Food Standards Agency
IMD: Inherited Metabolic Diseases
ICP: Intracranial Pressure
EFSA: European Food Safety Authority
BW: Bodyweight
DNSIYC: Diet and Nutrition Survey of Infants and Young Children
NDNS: National Diet and Nutrition Survey
ACMSF: Advisory Committee on the Microbiological Safety of Food
Technical Terms
5th Percentile Bodyweight: A statistical measure used to describe the lower end of a bodyweight distribution in a population. This means that 5% of individuals in the population weigh less than or equal to this value. Conversely, 95% of individuals weigh more than this value.
15th Percentile Bodyweight: This means that 15% of individuals in the population weigh less than or equal to this value. Conversely, 85% of individuals weigh more than this value.
25th Percentile Bodyweight: This means that 25% of individuals in the population weigh less than or equal to this value. Conversely, 75% of individuals weigh more than this value.
Low bodyweight: a body weight that is below the average or expected range for a person’s age, sex, and height.
Quantum satis: Quantum satis is defined in the legislation and means that additives shall be used in the food concerned in accordance with good manufacturing practice. This means that it must not be used at a level higher than is necessary to achieve the intended purpose and must not be used in a way that misleads the consumer.
Slushed ice drink: A beverage that is partially frozen, resulting in a slushy, icy texture.
Humectant: A substance used to keep things moist by attracting and retaining water from the surrounding environment.
Hypoglycaemic: Abnormally low blood sugar (glucose) levels.
Lactate: A compound produced during the breakdown of glucose in anaerobic metabolism.
Acute: Sudden onset, or of short duration
Pseudohypertriglyceridaemia: A falsely elevated level of triglycerides (fats) in the blood.
Hypokalaemia: Having low levels of potassium in the blood.
Glyceroluria: The presence of glycerol in the urine.
Gluconeogenesis: The metabolic process by which glucose is produced from non-carbohydrate sources, such as proteins.
Hygroscopic: A property of a substance that allows it to absorb moisture from the air
Osmotic: Related to how water moves through a barrier from less concentrated to more concentrated areas.
Genotoxicity: When chemicals damage DNA, causing mutations.
Intravenously: Administering something directly into a vein
Oedema: Swelling caused by fluid buildup in the tissues.
Osmotic diuresis: Increased urination due to the presence of certain substances in the fluid filtered by the kidneys, which causes water to be drawn into the urine.
Bolus/ oral bolus: A large dose of medicine taken all at once, either by mouth (oral) or into a vein.
Intraocular: Inside the eye.
Glaucoma: Eye conditions that damage the nerve connecting the eye to the brain, often due to high pressure in the eye.
Nausea: A symptom characterized by an uneasy stomach and the sensation of wanting to be sick.
Hyperosmolar non-ketotic coma: A serious condition with very high blood sugar, causing severe dehydration and confusion, but without ketones (chemicals that the body produces when it breaks down fat for energy).
Intracranial pressure: The pressure inside the skull affecting the brain
Acknowledgements
We would like to thank Professor James Coulson and Professor Shirley Price, members of the Committee on Toxicity, for their expert advice and contribution to this risk assessment. We would also like to thank Dr Aravindan Veiraiah and Dr Ellen Crushell, authors of the Brothwell et al (2025) paper, for providing additional information and advice.