Disclaimer
This report presents the key findings of the project FS900553/C302399 commissioned by the Food Standards Agency (FSA) and was conducted independently by Camrosh Limited. The main body of research for this report is based on secondary and primary research data gathered from academic and grey literature, and subject matter experts in fields relevant to this project including from academia, industry, and relevant governmental organisations in the UK and worldwide. Primary research data was gathered using an online survey and a workshop. In the primary research section of this report a range of views and opinions of participants were collected and presented, however these are not reflective of any specific individuals. Findings presented in this report are based on qualitative and quantitative primary research analysis as well as secondary research and are based on the authors’ analysis and interpretation of the findings. The findings, recommendations, and conclusions expressed in this report do not represent the view of the FSA and are not an expression of opinion on their behalf, nor an indication of priorities or future policy. Camrosh warrants that all reasonable skill and care has been used in preparing this report. Notwithstanding this warranty, Camrosh shall not be under any liability for any losses made by the client or its agents, and other related organisations, including but not limited to loss of profit, business, revenues, anticipated savings or indirect or consequential damage of any nature whatsoever, directly, or indirectly because of reliance on this report or of any error or defect in this report. Mention of names (e.g. firms, persons, products and organisations) is not an endorsement by authors or the FSA. Camrosh is not responsible for content from links and websites. The copyright of all materials in this publication rests with the respective content authors and expert contributors.
FSA Contract Reference: FS900553
Acknowledgements
We gratefully acknowledge expert advice and review by Prof. Lisa Conolly, Queen’s University, Belfast, and Prof. Michael Walker, Queen’s University, Belfast.
We gratefully acknowledge contributions by all participants to online survey and expert elicitation workshop as listed in appendix B
Date: 28/04/2025
Glossary of important terms
List of acronyms/abbreviations
Lay summary
Cultivated meat, also called cultured meat or lab-grown meat, is produced from muscle and fat cells grown in a laboratory by using technologies that are normally used for biological and medical research. The current mass production of meat using bred animals has many negative impacts on the environment and raises animal welfare and ethical concerns. Hence, cultivated meat might be a solution to produce meat in a different, more sustainable and ethical way. In the past three years the first cultivated meat products have been approved to be sold as food for humans in a small number of countries. Cultivated meat was allowed as ingredient for pet food in the UK in 2024. This report looks at one specific early step in the production process of cultivated meat, namely how the cells that are used as the starting material are produced and stored under special frozen conditions. This step is called cell banking and involves storing many hundreds or thousands of small tubes containing cells which can then be thawed to start the production process of cultivated meat.
This research has investigated published scientific research on cell preparation and cell banking, and leading experts in cultivated meat production and cell banking were invited to a workshop and consulted to find out what potential risks the early production steps including cell banking could pose for humans. Furthermore, this report discusses what food safety checks and tests are currently done by cultivated meat producers and what else could be done to ensure that cultivated meat is safe to eat. Insights on cell banking practices in the biomedical field are presented for comparison to what is currently known about cell banking in cultivated meat production.
The main insights from the research summarised in this report are the following:
-
We still have limited information about how companies prepare and store their cells before the main production process starts. There are no standardised rules and procedures yet for how to set up and manage cell banks for cultivated meat production. This is because using laboratory methods for meat production is a very new approach that was explored only over the past ten years at larger scale.
-
The technology will very likely develop and change rapidly over the coming years as the cells from the animals used for cultivated meat production have not been studied by science as much as cells from other animals (e.g. laboratory mice) which have been studied intensively with biomedical applications in mind. Researchers are currently still figuring out how to make the used muscle cells grow more efficiently and faster while being safe for humans to eat.
-
Cultivated meat producers are very aware of food safety risks and perform several tests required by food regulators. However, because different companies are currently using different production methods, there is a need for science-based guidance for this new industry. For example, it is not clear what tests for microbes and levels of leftover chemicals should be done, and how often along the lengthy production process these should be carried out
-
Currently, there are no specific rules or standard practices for the cell banking steps carried out by cultivated meat companies. But because the banked cells are the starting material for the final product, high standards might be required by regulators in the future to ensure a safe meat product of high quality. At the same time, experts consulted for this report agreed that future regulation of cell banking should not be overly restrictive so that companies can continue to innovate and improve the production process and their meat products, so they can also be commercially successful.
-
Methods for tracking the origin of cells and proving where cells have come from (e.g. from which animal, which supplier) are currently not well established. So far, no standard practices have become established across different companies. More science-based guidance on suitable tracking methodologies for cell banking and the whole production process are likely to be helpful for the new industry. Standardising such methods might also make product approval processes easier, so cultivated meat products can come to market quicker.
-
One concern about cultivated meat discussed in this report is that the cells might change their DNA over the course of the production process, which can take many weeks. The concern is mainly that such DNA changes might cause cells to make some molecules that can be harmful to humans. For example, molecules that some humans are allergic to. Experts consulted for this study think this risk is small, although specific scientific studies to understand the DNA changes that may happen during cultivated meat production and their potential impacts on humans have not been carried out so far.
In summary, while cultivated meat is a very new, fast-growing industry that takes food safety seriously, more research and clearer rules are needed to ensure that lab-grown meat is safe, trusted by consumers, and a successful alternative to conventional meat in the long term.
Executive summary
Cell Cultivated Products (CCPs) are novel food products intended for human consumption which are produced by laboratory techniques that were originally developed in the biomedical field. The rationale behind CCPs is the idea that directly growing nutritious cells in a laboratory might be more sustainable in terms of environmental impact, and address animal welfare as well as ethical concerns raised about current livestock-based meat production practices. The required laboratory technologies have over the past decade evolved and scaled, such that several companies are currently developing or offering CCPs in different parts of the world. Over the past three years a small number of CCPs, such as cultivated chicken nuggets (first approved in Singapore and the US) and cultivated steak (first approved in Israel) have been approved for human consumption. In 2024 the UK has allowed cultivated chicken meat as ingredient in pet food.
This report investigates specifically the cell culture and cell banking techniques currently used at the early stages of the production process in the emerging cultivated meat sector and related hazards and risks these techniques might pose to humans. Cell banking refers to the frozen storage of the specific cells that are the source for the production process. Here we have gathered the most recent evidence on the early stages of cultivated meat production processes including cell banking. Evidence was collected by conducting a comprehensive literature review of academic and grey literature, and a limited search for available patent information. In addition, an expert elicitation workshop was conducted with a range of academic and industry participants who had experience in relevant cell culture technologies and knowledge of related food safety aspects. The following summary presents the most salient findings of this study.
Currently, explicit information on cell banking procedures, cell banking media, and process ingredients used in a cultivated meat context is limited. As this is an area of emerging technology, no standardised definitions of master cell bank/working cell bank and related banking practices have yet emerged.
At present, early process stages of cultivated meat production are highly diverse and likely to evolve rapidly over the next decade. This is because, compared to cell lines from species traditionally used in biomedical research, cell lines being used for food production originate from species that do not have a long history of use in fundamental cell research laboratories. Hence, a considerable investment in innovation concerning cell optimisation, cell culture, and production techniques for use within a food production context is required. This includes genetically modifying cells to enable scalable and more cost-effective production processes.
Despite a diversity in approaches to producing cultivated meat products there is good awareness of general food safety issues and potential hazards impacting production processes among cultivated meat producers. However, the lack of standardised production processes creates a need for more evidence-based information and guidance on specific aspects of monitoring production processes (types of tests, statistical sampling criteria, frequency of testing, permissible minimal residue levels, among others).
Food safety testing and QC requirements specifically for cell banking in the cultivated meat context are currently undefined. However, experts consulted for this study agreed that a safe balance between setting high standards for the banking of source cells with technical as well as commercial feasibility for producers needs to be considered based on scientific evidence.
There are currently no defined standard practices or set requirements for cell origin and authenticity tracing. More specific guidance for traceability requirements might be necessary, along with additional guidance on potential future labelling requirements, to aid streamlining of product approval processes.
The role of genetic change/genetic drift of cells used in cultivated meat production as a source of potential risk for humans is at present scientifically not understood, although considered likely to be low by experts consulted for this study. Moreover, monitoring and testing requirements to establish degrees of genetic change throughout a production process are currently lacking. The scientific evidence base that would link genetic drift to risks for humans after consumption of genetically altered cells is highly complex, and currently no systematic studies on this matter exist.
Despite a diversity of approaches to cell culture and cell banking at early production process stages, and an expected dynamic evolution of the field, cultivated meat producers consulted for this study indicated that regulatory guidance on some aspects of production processes and final products would likely support industry growth and help with streamlining regulatory approval. Areas that might require more evidence-based information include toxicology and permissible residual levels of process chemicals and bioactive molecules in final products as well as clearer definitions of quality control (QC) criteria for cell banking practices.
1. Introduction
This report comprises two main parts: a review of the academic and grey literature in sections 2-6, and insights gathered from interacting with experts via an online questionnaire/survey and an expert elicitation workshop presented in section 7, with additional detail in appendix B. This introduction gives an overview of the rationale and context for this study.
1.1. Cell Cultivated Products (CCPs) and cultured meat - context
Over the past three decades, rapid scientific advancements in bio-medical research and applied biotechnology have enabled the direct use of a variety of cell types for therapeutic applications or the production of molecules of interest for various bio-medical/biotechnology applications. In addition, significant upscaling and commercialisation of many laboratory processes, now offered by a large biotechnology products and services sector, have led to increasing standardisation and reduction of cost in many application areas including cell line development and cell culture techniques. Although conceptually envisaged for many decades, the use of cell culture technologies that originate in biomedical laboratories to produce food for human consumption is now technically and commercially feasible. Cell culture-based food production applications fall broadly into two categories:
-
The production of specific nutritious proteins (e.g. milk, or egg proteins (Kwon et al., 2024; Nielsen et al., 2024), or other molecules that can be used as ingredients in different food products, such as fats (e.g. by Cultimate Foods), or flavouring and texture enhancing molecules (e.g. by Impossible Foods).
-
The manufacture of a finished food product. For example, a burger patty, a chicken nugget, or a piece of tuna sushi that consists of a large proportion of cells grown in vitro.
Based on this distinction, CCPs currently entering consumer markets are often categorised into:
-
Precision fermentation products. For these cell culture methods are mostly used at the beginning of the process to optimise and modify (often genetically) cell types that are then used as part of a fermentation process that produces the bulk of the biomass or specific molecules of interest. These molecular products of cells are then extracted and purified to create an ingredient for a more complex food product.
-
Cultivated meat and seafood products. In these the grown cells constitute a large proportion or most of the final product.
The focus of this report is exclusively on the latter, and specifically on the cell culture and cell banking techniques used at the beginning of the production process of cultivated meat products.
Over the past decade several cultivated meat and seafood products have been demonstrated, mostly by academic research groups and spinouts, with the first lab-grown steak using bovine cells reported in the media in 2013, followed by chicken and seafood products (Kirsch et al., 2023). Over the past five years regulators in a small number of countries have approved the sale of cultivated meat products for human consumption. Singapore approved a chicken nugget product manufactured by Eat Just and sold under its Good Meat brand in December 2020, and chicken products by Good Meat and Upside Foods received approval in June 2023 in the USA (The Good Food Institute, 2023). A beef product by Aleph Farm received approval in Israel in January 2024. The UK Animal and Plant Health Agency (APHA) and the Department for Environment, Food and Rural Affairs (DEFRA) have in July 2024 allowed a pet food product by Meatly that contains cultivated chicken meat which went on sale in January 2025 (Grierson, 2025). This makes the UK the first country in Europe to bring a cultivated meat product to market. In July 2024 the European Food Safety Authority (EFSA) has received its first Novel Foods application for a duck foie gras product by the French company Gourmey. The company has also applied for regulatory approval in the UK, USA, Switzerland, and Singapore, as they report in their blog post. Food regulators in other jurisdictions, such as Canada and Australia are currently considering cultivated meat products under their existing Novel Foods regulatory frameworks alongside dealing with application requests. As most companies developing cultivated meat products tend to pursue simultaneous applications for approvals in several countries, regulators need to monitor approval processes in other jurisdictions closely to understand how best to fulfil their own regulatory remits.
Major capital investments in research and development and the commercialisation of cultured meat production since 2019 (with an investment peak in 2021/22) have over the past five years significantly accelerated research and development increasing the number of players active in this field. Interest by large biotech companies as well as food industry players in shaping the emerging technology via collaborations with startups is expected to increase further in the coming years (The Good Food Institute, 2023). This increase in academic research interest and commercial and semi-commercial activities advancing cultivated meat production technologies was mainly driven by the motivation to find alternatives to conventional meat production processes which are known to have large negative environmental impacts and have been linked for a long time with animal welfare and ethical concerns. In this report we do not cover any of these aspects of cultured meat production in any detail.
The recent increase in research and development activity in cultivated meat and seafood is also reflected in the publication and patenting landscape analysed for this study (see Figure 1).
A similar dynamic can be observed in published patent documents.
Compared to other areas of biomedical and life science research the field is still small, thus the significant increase in activity over the past five years is more apparent. For search strings used in literature and patent analysis, and additional details see appendix A.
The rapidly growing ecosystem of academic and industry players is expected to generate more pronounced synergistic effects in the future with the main current efforts going into addressing specific issues around product quality and streamlining and scaling up of production processes to ultimately reduce the cost of cultivated meat products.
Despite significant media interest and attention, cultivated meat production is still in its infancy and available products are limited and expensive. Increased research and development activity, and first approvals of products by regulators over the past five years have helped to better understand the scientific and technical challenges of this emerging food production technology, encouraging active exploration of novel ways to address them. Further innovation is also an urgent necessity for this emerging sector, as it is generally acknowledged that current cultured meat products still lack taste and textural qualities that consumers would expect from these products to successfully replace conventional meat. As no “standard” products exist yet the study of nutritional profiles and possible longer-term nutritional and health impacts of such products is in its infancy (Fraeye et al., 2020; Olenic & Thorrez, 2023).
Much public and investor interest as well as positive media reporting has focused on the potential benefits of cultivated meat as it might help to reduce environmental impacts and address animal welfare and ethical concerns associated with conventional livestock farming. However, it should also be noted that in several countries there has been a public push-back against cultivated meat, often driven by farming lobbies. This has led to legislation banning cultivated meat in several US states, such as Florida, Alabama, Nebraska, and Missouri, with other US states debating such legislation (Flynn, 2024; Nowell, 2024). In Europe, Italy is in the process of legislating against cultured meat since 2023 and other countries such as Austria and France have raised concerns about cultivated meat with EU regulators. These initiatives were mostly driven by intentions to protect conventional livestock farmers from potential competition by the emerging cultivated meat producers (Bambridge-Sutton, 2024; Holland, 2024; Lanzoni et al., 2024).
These adverse reactions in some countries and a clear reduction in private investments over the past two years may generate some uncertainty in the future growth trajectory of cultured meat products. Growth estimates need to be considered within the general economic context of the food industry, which is characterised by low margins where profitability is always impacted by numerous factors such as: innovation specificity, indebtedness, company size, and reputation among other hard to predict market factors like volatile consumer sentiment (Grau & Reig, 2021). However, it appears that public funding and government initiatives supporting various biotechnology approaches for the food sector are likely to provide continuing support for the advancement of cultivated meat production processes in the near to mid-term future likely leading to more product applications to food regulators (Lanzoni et al., 2024; The Good Food Institute, 2023); (Vegconomist, 2025). As the technology is advancing, and more standardised production practices are developed and scaled up, regulators such as the FSA now have the opportunity to shape the future evolution of cultivated meat production processes by making regulatory decisions that not only ensure that products are safe for consumers but also help support further development of novel technologies that when more mature could bring tangible benefits to the food system.
1.2. Objectives of this report
In 2023 the FSA published a report on hazard identification in meat products manufactured from cultured animal cells (Smith-Uchotski et al., 2023). It provided a first assessment of potential hazards relevant to food safety that might arise from the known production steps involved. This present report focuses on the early process stages of production from cell sourcing and isolation, cell line establishment and cell banking prior to the day-to-day production steps that will give rise to the bulk of cells and the finished meat product. While it is understood that various hazards might be introduced at any of these downstream process steps, it is also clear that from a QC point of view the cell bank plays a central role in ensuring food safety and product quality. The cell bank is the source from which cells are taken for use in production, giving rise to all cells that are produced in downstream processes, and which form the final product.
The main objectives of this research project were the following.
-
To provide a comprehensive and up-to-date overview of available information on cell isolation, cell line establishment, and cell banking practices currently used by cultivated meat producers and related academic research.
-
To provide an analysis of potential hazards as well as prevention and mitigation strategies that are currently understood and implemented in the early process stages up to cell banking.
-
To provide a comparative analysis of cell banking practices for biomedical applications, hazards, testing requirements, and mitigation strategies to fulfil regulatory requirements.
-
To assess the current understanding of hazards, QC, risk mitigation strategies, and regulatory requirements among academic and industry experts in the emerging cultivated meat sector. This will identify areas in which regulatory guidance might be perceived as helpful for the evolution of the emerging cultivated meat sector.
Findings from the literature review and the expert elicitation workshop are combined and integrated to inform conclusions and recommendations of this report. However, findings, conclusions, and recommendations are those of the authors of this report and intended as information for the FSA to help with establishing the evidence base on cell banking practices in the cultivated meat sector, but do not reflect opinion or policy intentions of the FSA.
1.3. Methodology - overview
Literature review
A comprehensive literature search was carried out using the lens database for academic literature and patent data. Several search strings were used to cover overall publication trends and publication frequencies of highly active authors over the past two decades. Researcher-led analysis of titles and abstracts was used to identify most relevant literature for details on cell banking in the cultivated meat context. In addition, literature from relevant fields of biomedical research relating to cell banking practices for biomedical applications was explored with regard to cell banking practices. Patent documents explicitly referring to innovations for the production of cultivated meat or other forms of cellular agriculture were retrieved from the lens database and analysed for relevance to cell banking practices and patenting trends over time. In addition, open-source web search was carried out to retrieve relevant industry publications and regulatory documents. For details on search and additional data on literature and patent documents, see appendix A.
Expert elicitation workshop
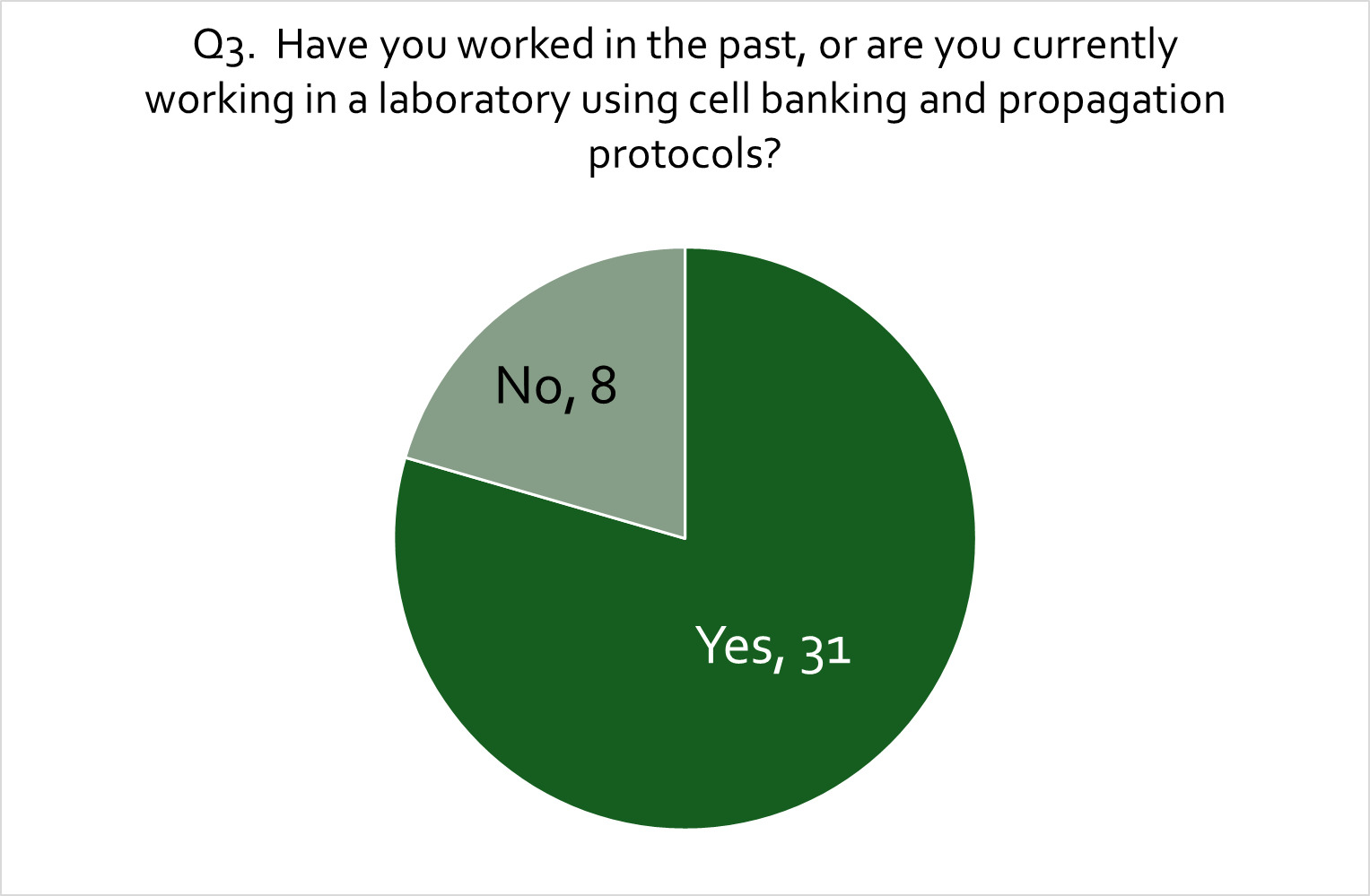
An expert search was carried out globally using open-source information, ensuring a good international spread of academic and commercial expertise. A range of different cultivated meat producers was contacted representing different product categories separated by species of origin of cells including cattle, chicken, and fish and seafood products. In addition, care was taken to include companies covering a range of technological and commercial maturity, and experts at different levels of seniority. One hundred and forty-five relevant experts were initially contacted and requested to participate in the workshop and to complete a pre-workshop questionnaire/survey. The questionnaire was intended to better gauge the area and depth of expertise of potential workshop participants and to get an initial understanding of the specific potential food safety hazards along the production process, whether there are specific critical steps/process stages that needed to be considered, and what role regulation might play for this emerging food production technology in the opinion of consulted experts. Of the 145 experts contacted, 39 returned a completed questionnaire, and 19 took part in the online workshop. For details and data on the questionnaire and expert elicitation workshop see Appendix B.
2. Cultured meat production process - overview
Although a general, “standardised” workflow for cultivated meat production does not currently exist, several fundamental steps are required in most approaches. However, it needs to be considered that each of these individual workflow steps might undergo substantial modifications in the near future to make production more efficient and commercially viable. Moreover, the exact technical details of different workflows can vary considerably and are often protected by intellectual property (IP) law. For the purposes of this report the focus of this overview will be on the early process steps as these are most relevant for cell banking practices.
Figure 3 depicts a generalised flow diagram of the main production steps for cultivated meat as it is often presented in the academic literature. Similar simplified workflow diagrams are also presented on the websites of cultivated meat companies (Reiss et al., 2021).
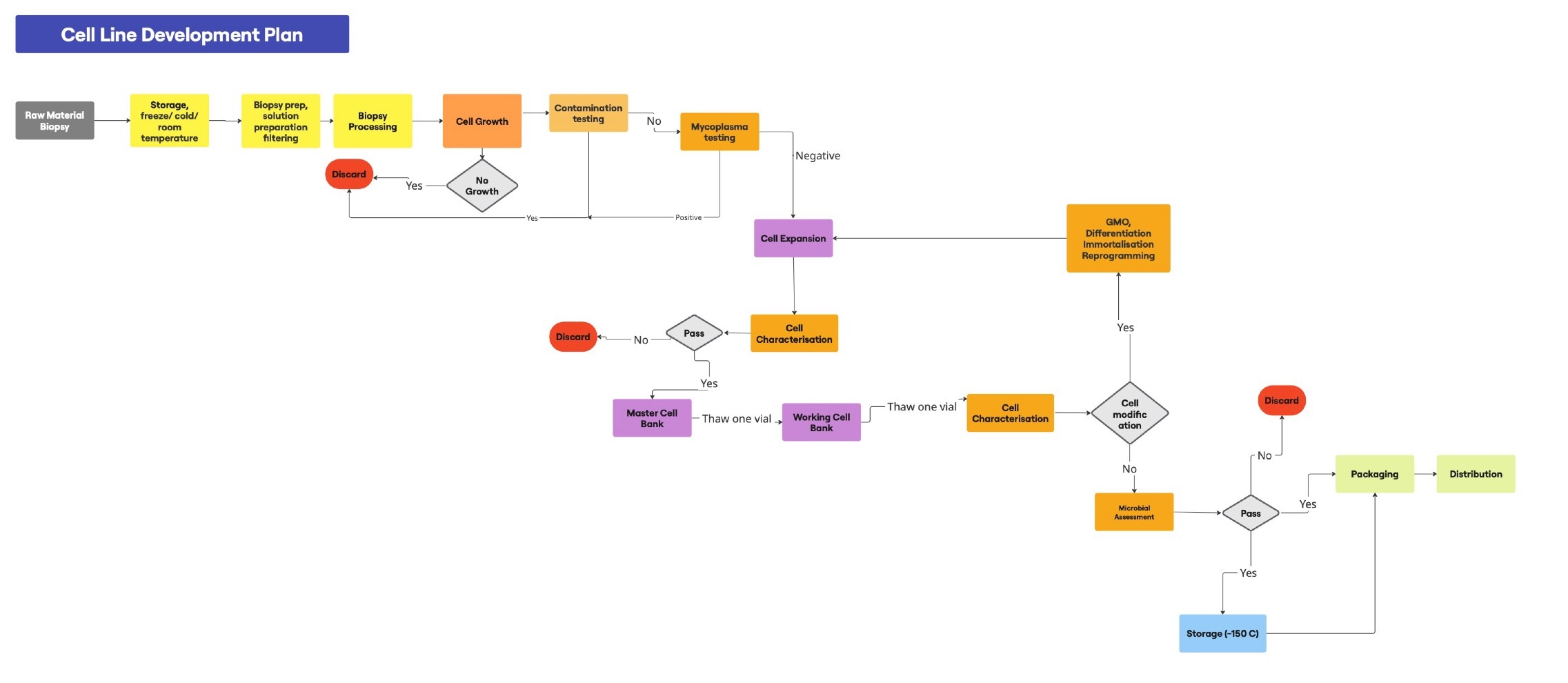
A representation of a cultivated meat production process workflow including an initial cell banking step is shown in Figure 4 (Hauser et al., 2024).
The composition of skeletal muscle is known to comprise approximately 90% muscle fibres, 10% fat, connective tissue, blood vessels, nerves, and less than 1% blood. Hence, the main input source cells into various cultivated meat production processes are muscle and fat cells (Listrat et al., 2016). However, the techniques for utilising fat cells are less well developed than for muscle cells. The following fundamental steps presented in sections 2.1-2.4 apply to most protocols for cultivated meat production processes including cultivated seafood.
2.1. Selection of source/input cells
Adult muscle tissue contains a proportion of “reserve” stem cells, called satellite cells, that can differentiate into muscle fibres in case of muscle injury to repair damaged muscle areas. Hence, adult muscle tissue is a common source of such muscle stem cells. As fat contributes an important component of meat flavour, fat cells are the second cell type that most cultivated meat producers are currently aiming to include into their products. To isolate adult resident muscle or fat stem cells, most commonly a tissue sample is collected by performing a biopsy either from a live animal or postmortem, immediately after slaughter. For some animal species such as cattle careful donor selection (age, sex, breed) as well as biopsy location within the body have been shown to impact final muscle stem cell yield and proliferation potential. Different biopsy techniques have been tested for cattle and other species and can routinely yield between 0.5g of tissue material for standard needle biopsy and up to 15g when using incision biopsy techniques (Melzener et al., 2021). It appears well acknowledged in the cultivated meat field that for both procedures various regulations need to be adhered to with regards to food hygiene, including hazard identification, critical control points, critical veterinary residue concentration limits, testing and monitoring of relevant food hygiene parameters, record keeping, and verification practices common to other food production processes (Melzener et al., 2021). In addition, relevant animal welfare parameters relating to biopsy procedures such as degree of discomfort and stress during immobilisation and sedation in a cage, length of time of tissue regeneration after biopsy, and degree of pain experienced by the animal, among others have been studied in the past and are reasonably well understood and seem to be considered when sourcing cells from animals via biopsy procedures (Melzener et al., 2021; Mølgaard et al., 2012). It should also be noted that taking biopsies from animals is regulated in the UK by the Animals (Scientific Procedures) Act 1986 and is hence a procedure that needs to follow specific protocols and documentation. Alternatively, for chicken meat products, fibroblasts can be harvested from fertilised chicken eggs, which are subsequently immortalised and used to generate muscle and fat cells through a trans-differentiation process controlled by various growth and differentiation factors (see below). Many other alternatives for initial cell sourcing to obtain relevant stem cells are currently actively being explored and tested (Ma et al., 2024).
The collected tissue sample is then processed to isolate the relevant tissue specific Adult Stem Cells (ASCs) namely muscle satellite stem cells that can differentiate into mature muscle cells and fibres, and the fibro-adipogenic progenitor cells (FAPs) that can differentiate into fat cells and give rise to most of the fat in muscle tissue. Other options for fat cell production have been successfully tested from mesenchymal fat and bone stem cells as well as trans-differentiated muscle cells (Cortes et al., 2013; Zagury et al., 2022).
Given that sourcing ASCs from biopsies might require repeated biopsy procedures once a production facility runs at scale, batch to batch variations in overall cell quality and their proliferative as well as differentiation potential are expected and may impact downstream processing and final product quality. Although the ASC route is currently the most widely used approach, its limitations in terms of variability and the need for repeated regulated procedures on animals make other types of stem cells with indefinite proliferative potential and flexibility in differentiation pathways highly attractive for cultivated meat production. These stem cell types are:
Embryonic Stem Cells (ESCs)/Pluripotent Stem Cells (PSCs) and induced Pluripotent Stem Cells (iPSCs), all of which are being currently tested and optimised in various academic and commercial research groups for cultivated meat production. PSCs are isolated from the inner cell mass of early-stage mammalian embryos when they consist of only up to a few hundreds of cells and can differentiate into any tissue specific cell type given the right external molecular environment, tissue specific growth factors and instructive signalling molecules are provided. iPSCs can be derived from tissue-specific adult stem cells (harvested from an adult animal) by exposing them to a set of specific transcription factors that “re-program” (induce) them into an “embryonic”, pluripotent state. These reprogramming factors are SOX2, OCT3, OCT4, KLF4, and cMYC, also called Yamanaka factors after one of the Nobel laureate scientists who elucidated their role in cellular reprogramming (Liu et al., 2008). Subsequent exposure of iPSCs to the right instructive signalling molecules and extracellular environment can result in directed differentiation of almost any tissue-specific cell type (Cerneckis et al., 2024). Currently, skin fibroblasts are the most commonly used cells for reprogramming iPSC approaches (Ma et al., 2024). The main routes of obtaining stem cells for cultivated meat production are shown in Figure 5 below (Kulus et al., 2023).
As ESCs, and iPSCs have a capacity for almost unlimited proliferation and are amenable to genetic modification to optimise them for the cultured meat production process they are widely seen as the most flexible and promising source cells for the future. However, there are at present several technical challenges with regard to upscaling and consistency of molecular reprogramming protocols (Martins et al., 2024; Reiss et al., 2021). The establishment and species-specific optimisations of stem cell culture protocols, e.g. for porcine and bovine muscle and fat cells, is a rapidly growing field with stem cells of various species currently being tested by academic and commercial research groups with some success (Bogliotti et al., 2018; Cheng et al., 2023; Gao et al., 2019; Zhu et al., 2023).
Immortalised cell lines are another type of input cell actively pursued for cultivated meat production. Immortalised cells have been widely used for biomedical applications for the past 50 years due to their indefinite proliferation potential. Immortalisation of cells can either occur spontaneously in cell culture under certain conditions or be introduced through genetic engineering that disrupts cell cycle regulators and causes aberrant telomerase function that enables indefinite maintenance of chromosomal telomerase length, resulting in indefinite proliferation potential like in a cancer cell. A large range of human and mouse immortalised cell lines have been widely used and optimised via genetic engineering techniques for various bio-medical applications in the past, including muscle cells for therapeutic applications (Pawlowski et al., 2017). In addition, a large ecosystem of academic and commercial producers and suppliers of such cell lines exists. At present, several research groups are working on identifying and genetically optimising relevant immortalised cells from different species of interest for meat production, such as for example porcine preadipocytes (Cheng et al., 2023).
For an overview of tested routes to source cells currently being pursued for cultured meat production alongside their advantages and disadvantages see Figure 6.
2.2. Stem cell enrichment/expansion – cell line establishment
Once the source of stem cells is established, it is essential to generate large quantities of stem cells with high proliferation potential. These qualities are essential for generating the master cell bank and subsequent working cell banks that will be used in production runs. In the case of biopsy-derived cells, this involves the mechanical and chemical disruption of tissue and isolation of the stem cell population, usually followed by an enrichment step in which stem cell specific antibodies are used to label the stem cells of interest. As antibodies to most stem cell markers were initially developed for mouse and human cells, the search for relevant markers of the stem cell state in species relevant for meat production is an increasingly active research field as marker options are currently limited for many species (Reiss et al., 2021).
Commonly, Fluorescence-activated Cell Sorting (FACS) is used to selectively collect only the stem cells of interest from a tissue biopsy sample (Maesner et al., 2016). After a reasonably pure portion of stem cells is collected via FACS sorting, an “expansion” or proliferation step is required to produce large quantities of stem cells. Depending on the type of source they are derived from, they will have very specific requirements in terms of molecular constituents of the growth medium as well as the extracellular matrix (ECM) environment to promote proliferation. Stem cells in their natural tissue environments maintain their “stemness” through continuous interaction with the three-dimensional tissue “niche” in which they reside and its respective extracellular matrix molecules. Hence, the exact composition and mechanical properties of the first expansion growth environment are crucial for obtaining satisfactory stem cell yields. This also poses a technical challenge for upscaling approaches, as bioreactors and suspension culture are the most feasible growth environments of choice for economic reasons, but not ideal proliferation environments for stem cell expansion. For example, cells can be damaged due to shear forces resulting from various mechanical stirring and shaking mechanisms which are required in larger bioreactor or bottle cell culture systems. As stem cells are not adapted to grow in such environments various ways are currently being explored to genetically engineer cells in such a way that they do not rely as much on specific biochemical and mechanical ECM substrate parameters and are more resistant to shear forces (Kulus et al., 2023).
Having generated enough stem cells, a first master cell banking step is carried out. This step involves usually a thorough characterisation of harvested cells using morphological phenotype analysis by microscopy as well as genetic marker and cell type-specific antibody testing. These tests should confirm the expected correct identity of cells and their normal proliferative properties. Once these tests were satisfactory, cells are aliquoted and frozen in master cell bank vials containing a storage medium and cryoprotective additives which reduce damage to cells upon freezing. Individual vials from the master cell bank are then used to further multiply stem cells which are then stored in multiple vials of a working cell bank. Further downstream process steps for day-to-day production are usually carried out using cells from the working cell bank.
2.3. Differentiation
After initiating a production run using starter cells from an established working cell bank and once the stem cell population has reached its desired density, cells are exposed to the relevant molecular environment that triggers differentiation into mature muscle and fat cells that can then be harvested for further downstream processing steps. This involves growing the cells in a different type of bioreactor and culture medium that contains all the growth factors and signalling molecules that induce differentiation into adult muscle or fat cells. One example depiction of muscle differentiation is shown in Figure 7 below, along with the protein expression activity of the relevant signalling pathways that control the respective differentiation step.
While such depictions imply linear transitions from one differentiation step to the next, it needs to be considered that each step requires specific external cell culture parameters to be optimised to achieve good yields of each of the differentiation stages. In particular, the organisation of individual muscle cells into myotubes and further into mature muscle fibres requires the right molecular and biomechanical growth substrates as natural differentiation requires continuous signalling interaction with the specific ECM molecules that normally support differentiation. Hence, various growth substrates such as hydrogels, engineered matrices e.g. made of collagens and other basement membrane proteins on 3D porous structures, or various microcarriers that can be used in suspension culture in bioreactors are currently being tested. This includes various plant or animal-based porous substrates and self-assembling peptides which are currently being tested with a view to upscaling the differentiation stage of the production process with regard to final product assembly and texturization (Bodiou et al., 2020; Bomkamp et al., 2022).
Figure 8 shows the major growth substrate dependent process steps required to produce cultivated meat products.
Growth substrates are essential for initiating the correct cell differentiation pathway during the large-scale cell expansion stage. However, if they are not suitable for human consumption in larger quantities, a trade-off between methods to remove them at the end of the expansion process and choosing edible materials needs to be considered. In most current cultivated meat production approaches growth substrate is an unavoidable constituent of the final product. At present, many biotechnology companies and academic research departments are spending considerable efforts to develop and optimise various scaffolding materials that are compliant with food regulations, specifically for cultivated meat production. Bomkamp et al., 2022 describe in some detail the composition of several classes of currently used and tested scaffolding materials. These, non-exhaustively, range from synthetic polymers such as polystyrene, methacrylate, acrylamide derivatives, proteins of animal origin such as collagen, gelatine, hyaluronic acid, whey protein, or molecules of plant origin such as gums, cellulosic materials, textured vegetable proteins, or components of seaweed, algae and fungi. Advantages and disadvantages of different scaffolding materials are shown in table 1 below.
Once sufficient quantities of differentiated cells have been produced, they need to be grown further on another 3D substrate to enable final processing and texturizing in a final maturation and processing stage.
2.4. Maturation and product processing
The level of technical complexity required of biotechnologically developed scaffolding depends on the desired properties of the final cultivated meat product. In cases such as “minced meat patties” or meat paste (such as cultivated foie gras) where final structure does not matter as much, growth of fused primary muscle fibres in flat layers on a suitable substrate might suffice, and fat cells can be grown in a separate workstream and added in a non-structured manner. In cases where a more structured product is, for example, intended to mimic a cut of meat, considerably more technical effort needs to be spent on final product assembly using additional texturizing growth substrates. In addition, various 3D printing approaches are currently being tested to deliver cell adhesive as well as specific textural characteristics to cultivated meat products including the use of plant derived materials (Albrecht et al., 2024; Ianovici et al., 2022; Su et al., 2023; Wang et al., 2024). As the current cultivated meat products that have entered consumer markets are still considered unsatisfactory in terms of texture and taste, the engineering of final texturizing approaches is a highly active area of research and development in the emerging cultivated meat sector. However, for the purposes of this report we do not cover this stage in any detail.
3. Hazards and risks – current understanding and relation to cell banking practices
With the first approvals of cultivated meat products by regulators, a better understanding of which potential hazards and risks must be addressed by producers from a food safety perspective is now emerging. A body of literature on potential hazards in the cultivated meat sector has developed over the past five years and published risk/hazard analyses have taken the whole of the production process into account. Several studies have recently reported on the most salient food safety issues also with a view to compliance with regulatory frameworks in different jurisdictions (Lanzoni et al., 2024; Powell et al., 2025; Zandonadi et al., 2025). Moreover, relevant risk assessment documentation from product approval applications and pre-market notice documents are now widely shared and likely used to streamline Hazard Analysis and Critical Control Points (HACCP) protocols to align with food safety standards for certain countries. Examples of such publicly accessible documents are the applications of Good Meat for a cultivated chicken product and the premarket notice document for a chicken product by Upside Food, with another application for cultivated pork fat cells submitted to the Food and Drug Administration (FDA) in the US by Mission Barns in March 2025.
In addition, large biotech industry players, such as Merck, supplying the emerging cultivated meat sector have started to provide guidance documents on good production practices.
Since a previous hazard assessment report published by the FSA in 2023 (Smith-Uchotski et al., 2023), a number of academic and industry publications have specifically analysed various biological and non-biological hazards of cultured meat production, mostly corroborating the findings of the FSA report. See table 2 as an example from a publication by a bio-pharma products supplier (Merck, 2024).
With more information available to regulators from recent approval applications, the requirements of specific testing points throughout the production process following strict protocols of Hazard Analysis and Critical Control Points (HACCP), Good Manufacturing Practices (GMP), and Good Laboratory Practice (GLP) are becoming better understood. As an example, see below table 3 with identified hazards of raw input materials of external origin, focusing on biological hazards.
Moreover, large international organisations such as the WHO and FAO have investigated food safety aspects of CCPs and published detailed risk analysis tables with the major production stages defined for risk assessment purposes as shown in Figure 10 below (FAO, 2023).
According to this categorisation, risks emerging from cell banking practices are emerging at stages 1 and 2, which is well aligned with categorisations used in other academic literature.
With more players active in the field, more studies are investigating specific hazards of cultured meat production, their actual prevalence and related current testing practices. For example, outcomes of a survey of cultivated meat producers indicate that most companies tend to carry out more microbial contaminant testing at later process stages, such as during bioreactor differentiation and cultivation, on wet cell mass post-harvest, and final product stages (Powell et al., 2025). The most common contaminants reported in this study were bacteria and fungi. Interestingly, the main contamination vectors were reported as mainly due to exposure during cell harvest and improper sterilisation of equipment, followed by contamination through media ingredients and cell bank contamination. Importantly, only a minority of respondent companies in this study reported to have a HACCP plan in place at the time of survey, which may explain some of the detected patterns of contamination (Powell et al., 2025). Similar results on most frequent contamination risks relating to initial cell sourcing via biopsy procedures were also reported by Food Standards Australia New Zealand (FSANZ) in relation to an application for approval by a cultivated meat company.
Several main vectors and entry points of biological contaminants into the production process are well documented and agreed upon in the academic and grey literature. These are presented in section 3.1 below.
3.1 Microbial vectors
Vectors of zoonotic diseases and foodborne pathogens can be transferred via several routes either from the source animal or its immediate environment during biopsy procedures, and via downstream handling of cell material. In particular, bacteria and viruses such as endogenous retroviruses, species-specific viruses, or viruses entering cell cultures from handling and other laboratory cross-contaminations have been identified in other laboratory-based manufacturing contexts and would also apply to the cultivated meat sector (Barone et al., 2020). However, overall contamination risks of cell sourcing for cultivated meat production were sometimes reported to be lower than in conventional meat production (Treich, 2021).
Antibiotics are commonly used to reduce contamination risks from source animals during biopsy collection steps. Specific veterinary inspection protocols for source animals before and after biopsy are recommended in the literature to reduce the risk of contamination with adventitious agents from donor animals. However, for some CCPs such as cultivated sea food no such standardised procedures are established (Melzener et al., 2021). (As already mentioned above, taking biopsies from live animals is in the UK regulated by the Animals Scientific Procedures Act 1986). Moreover, cells and cell lines obtained from repositories and commercial providers were reported to be least contaminated compared to other sources as they are usually rigorously tested prior to and during the cell banking stage (Powell et al., 2025). Hence, cultivated meat producers might be expected to increasingly use cells from such sources as they become more commercially available. A more detailed description of biological contaminants and testing requirements relevant for cell banking and cell culture will be covered in section 5.
3.2. Culture media and components of animal origin
Most culture media currently used in the initial process stages up to cell banking still contain animal-origin ingredients. Examples include Foetal Bovine Serum (FBS), Bovine Serum Albumin (BSA) (although BSA is also available as recombinant protein at higher cost), and certain hormones. Hence, substantial research and development efforts by producers and academic research groups are currently dedicated to developing and testing animal-free, chemically defined media with some success; most producers appear to be committed to producing meat in media without animal-sourced ingredients in the future (Kolkmann et al., 2022; O’Neill et al., 2021; Stout et al., 2022).
However, a rapidly expanding market of novel media and ingredients that should replace animal-sourced ingredients may give rise to other concerns. For example, due to so far untested allergens and ingredients that are not disclosed for IP protection reasons. At present most animal-free media compositions are not yet as efficient in terms of proliferation rates and successful differentiation of cells (Ong et al., 2021). Biological vectors/pathogens being carried over from the initial cell banking stage therefore remains a risk, because the very first laboratory-scale stem cell enrichment steps after sourcing of cells from biopsies and prior to first cell banking still appear to be carried out mostly in conventional media. These usually contain animal-sourced components and animal sourced growth substrates to ensure good initial stem cell yields before selecting cell batches for banking. For example, initial stem cell enrichment steps prior to cell banking require the use of serum and growing cells on ECM substrates and attachment factors such as collagens or other mixtures of natural extracellular matrix proteins sourced from animals (e.g. as sold under the trade name Matrigel®, which is derived from cell culture supernatant of murine osteosarcoma cells) (Lee et al., 2021).
3.3. Growth factors and hormones
Growth factors and hormones are essential components of media during all production stages, including those prior to banking. A number of growth factors such as insulin-like growth factors (IGF-1, IGF-2), transforming growth factor beta (TGF-β), fibroblast growth factors (FGF-2 and FGF-21), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), and hormones such as insulin, glucocorticoids, testosterone, and thyroid hormones are required for efficient development of muscle cells. Fibroblast growth factor 2 (FGF-2) is routinely used in all early isolation and expansion steps (Ahmad et al., 2023; Lee et al., 2021). In addition, it has been reported that in the case of iPSCs that residual re-programming transcription factors might carry over into downstream processes. In approaches where these are provided as proteins in the re-programming culture medium these are often human-derived, and some, like cMYC would be more difficult to remove completely, although amounts are likely to be low and diluted during further downstream processing (Szeder et al., 2024). Currently there are no specific analytical methods proposed to routinely test for such residual bioactive molecules.
Residual levels of such bioactive molecules are of concern in the final product and removal, or deactivation must be considered before product shipment as many jurisdictions have strict regulations which biologically active substance must not enter the food chain as for example outlined by EU regulation for farm animals; (Lanzoni et al., 2024). The absolute concentrations of such molecules carried over from the banking stage can be considered small compared to levels potentially found in downstream steps and processed product. However, contamination with pathogens can arise when such hormones and growth factors are derived from animal sources, as with any other animal sourced ingredient. Increasingly, species-specific and more thermostable recombinant hormones and growth factors are being used and offered by several companies supplying the CCP market (e.g. Qkine).
3.4. Genetic drift
Random genetic change is a natural phenomenon that occurs in all cells of an animal over its lifetime as well as in cells passaged in cell culture. Within a population of organisms, or a population of cells in culture, such genetic changes can change the frequency of the initial average genotype within the population over time. This phenomenon is called genetic drift.
Genetic drift over time is caused by the emergence of random genetic mutations along the DNA, chromosome rearrangements and other genetic and epigenetic processes. These genetic changes (mutations) can either lead to cell death (if the change has a detrimental effect on cell survival) or in some instances to subtle or more substantial change in cell physiology and phenotype (Ben-David et al., 2018; Cao et al., 2021). In an organism these genetic changes occur randomly over time, or due to some external factors (mutagens), such as ionising radiation, exposure to toxins. Such external influences can also increase the rate at which genetic change (mutations) occurs. Such “somatic” mutations can sometimes lead to abnormal cell types that in most cases are removed by the animal’s immune functions. In cases where such cells can evade immune detection, cancer may develop.
In cell culture, such genetic drift may be induced at higher frequency due to stress conditions in the cell culture environment, such as increased metabolite concentrations, lack of oxygen, or bacterial and fungal contamination among others (Attwood & Edel, 2019; Li et al., 2019; Ong et al., 2021). As final product quality and consistency of CCPs depend on a specific well-defined cellular genotype and phenotype, monitoring genetic variation during the early expansion stages before cell banking is essential. Moreover, when genetically engineered cells are used, the integrity of the engineered genetic elements needs to be ascertained before cell banking stages to ensure their traceability.
Random genetic changes may in some cases induce cells to express proteins either at different levels or with different or novel characteristics. Such proteins or a significant change in their expression level might affect human physiology in some instances and may include allergens and toxins, while not affecting the cultured meat production process. However, the current literature on such cases is limited (FAO, 2023). Hence, whole genome sequencing (WGS) and karyotyping of source cells at the cell banking stage would be essential to ensure that early processing steps, such as induction of the stem cell state (in the iPSC approach) or enrichment/expansion of stem cells do not introduce consequential genetic alterations before banking of master and working cell banks. In addition, other tests to ensure that cells have maintained their stem cell state prior to banking might be required (Ben-David et al., 2018). Regular genetic screening of working cell bank batches might also be necessary to meet possible future regulatory requirements for cell origin tracing and authenticity labelling. In jurisdictions where stricter labelling requirements for food products based on genetically modified cells exist, regular genetic assessment of the input cell types used might be relevant. Some explicit considerations regarding authenticity testing for cultivated meat with similar analytical DNA and protein assays methods as currently being used for conventional meat have been suggested, which again might involve also the detection of deliberately introduced genetic modifications which are expected to be part of engineered cells (Mariano et al., 2023).
3.5. Cross-contamination with foreign cells or altered cell types
Cross contamination with cells is a very common and well-documented phenomenon since the early days of cell culture practices (Weiskirchen et al., 2023). This affects any type of cell culture, and the cell banking stage is one of the most crucial stages when this can occur. In the case of source cells for cultivated meat production, two types of contamination need to be considered: i) contamination with foreign cell types from the laboratory environment (e.g. human cells) via handling and bad laboratory practice and ii) contamination with altered cell types through loss of intended stem cell state or genetic drift induced during the early process stages immediately prior to banking. These may introduce cell types that may not harm the production process but might grow to a substantial proportion of the final cell culture mass impacting on final product characteristics. In the cultivated meat production workflow, however, such events might be considered more likely to occur than in biomedical applications given the large scale of cell culture processes and the large numbers of cells involved. In addition, source cell material is either already heterogeneous as in the case of biopsy derived cells, or the differentiation/stemness state in iPSCs might become unstable due to random fluctuations in the culture conditions and become differentiated into cell types not intended to be part of the product (Ong et al., 2021).
3.6. Cell misidentification
Cell misidentification is sometimes caused by undetected cross contamination with other cell types but often also the result of human handling and recording errors or bad laboratory practice. This is also a well understood issue in any industry that uses large cell banks or biobanks and rigorous procedural protocols for data capture and process documentation in cell banking are required as is the case in other applications of stem cells for example in the biomedical Genetic and Cell Therapy (GCT) sector. International standards and regulations exist in this area (Mah et al., 2023; Souren et al., 2022). See also section 5 on banking of stem cells for biomedical applications.
4. Reporting on current cell banking practices in cultured meat production
As of writing of this report, literature with an explicit focus on the cell banking stage of cultivated meat production at a detailed technical level is currently lacking. However, potential risks and hazards that can be introduced prior to banking are reasonably well discussed in the academic literature and industry publications, as outlined in sections 2 and 3 above. Using extensive search strings to search specifically for publications on cell banking in the cultivated meat context (for details see Appendix A) retrieved only 13 publications none of which contained specific relevant information on cell banking practices.
The basic steps of establishing cell banks are assumed in most publications as a routine laboratory process, well understood from other areas of cell use in biomedical applications. The main steps reported in the cultivated meat literature are therefore often summarised as follows.
Relevant source input cells or already characterised cell lines may be obtained via different cell isolation routes or acquired from commercial and other sources. These cells are frozen in aliquots according to different cryopreservation protocols using various cryoprotectant additives. Such aliquots represent the “master cell bank”. However, cell banking is also routinely performed at different early and later downstream stages, such as after the initial stem cell enrichment/purification and expansion of individual stem cell types (FAO, 2023; Ong et al., 2021; Tan et al., 2024). Aliquots frozen and systematically banked at such downstream stages are then considered a “working cell bank”. Cells taken from a working cell bank are understood to be of the desired type and purity to start large scale proliferation/expansion processes. These are then followed by a final harvesting step that provides the cells for final product “assembly” and texturizing processes that generate the final product format (e.g. meat paste, layers, patties, “chicken nuggets” among others). The understanding of “working cell bank” appears currently to be that vials from the working cell bank contain the cells that would start an actual production run of cultivated meat manufacture, equivalent to a run of experimentation in biomedical research (Healy et al., 2011).
From current reporting it is not clear whether additional cell banking steps are carried out at specific downstream process steps for batch quality control and documentation purposes. However, depending on the input cell type, and whether cells were produced in-house or commercially acquired, what is considered the master cell bank might vary. Hence, a clear definition framework for what is considered a “master cell bank” and what a “working cell bank” in the cultivated meat sector might be useful to establish with regard to the clarity required for regulatory decision making.
It is anticipated that once cultivated meat production processes reach commercially viable scale, the required cell banking practices would also need to reach unprecedented scales, including the continuously required associated QC testing regimes. Such transformational upscaling of cell banking practices has been explored for other stem cell-based applications such as the mass production of off-the shelf cellular therapeutics, and is anticipated to be much bigger for the cultivated meat sector in scenarios where cultivated meat production would replace a significant proportion of conventional meat production (Martins et al., 2024; Melzener et al., 2021; Woods et al., 2016).
Hence, the benefits of centralised and commercial access to safe well-characterised and traceable cells are being actively explored to reduce uncertainties, regulatory burden and hazards due to cell banking requirements. Several commercial players are already offering cell banking services (e.g.: Benemeat,, Extracellular). Further collaborations and supplier ecosystem synergies between companies manufacturing novel (animal product-free) media and cell providers offering cells and banking services tailored for cultivated meat production might help consolidate and simplify cell banking for producers in the future as advertised by one UK supplier.
Although companies may benefit from in-house IP and proprietary innovations, part of the sector already appears to promote more centralised and standardised access to safe cells (Bennie et al., 2025; Ravikumar & Powell, 2023).
Moreover, as unmodified mammalian cells are not adapted to various stress factors of industrial scale production processes, genetically engineered cell types that are optimised for cultivated meat production are expected to be a prerequisite for industrial upscaling and standardisation of production processes. Specific genetic modifications are being actively explored and tested currently to achieve goals such as life-span extension/immortalisation, increased proliferation rate, cell intrinsic genetic control of differentiation (instead of adding molecular factors to the medium, so called “forward programming” genetic modifications are engineered into the cells), substrate independent growth (tolerance against shear forces in suspension culture in bioreactors), and properties affecting media requirements in order to be able to use cheaper and less complex media, among others (Keller et al., 2014; Pawlowski et al., 2017).
Given the complexities involved in establishing such engineered cell lines, commercial and more centralised cell sourcing and banking practices may ultimately become more widely used with increasing commercial availability of such cell lines. This might also help consolidate cell banking practices for cultivated meat producers (Riquelme-Guzmán et al., 2024). Risk-based approaches for a more centralised provision of banked safe cells at a large scale to reduce and mitigate risks are already actively being investigated building on experience from other cell banking application areas (Bennie et al., 2025).
Although attempts are currently made to develop “basic” protocols based on most-commonly used laboratory protocols and media ingredients for a “generic” cultivated meat production process, cell banking steps are not explicitly included, as shown for example in Figure 11 below (Lee et al., 2021).
As explicit reporting on cell banking practices in a cultivated meat context is currently limited, we present insights from cell banking practices for biomedical applications in the following section 5.
5. Insights from stem cell banking for biomedical applications
In this section an overview of current practices in stem cell banking for biomedical applications is presented to provide a comparison at the technical level. However, when reporting on recommended practices in this field, it is not intended to imply that such recommended practices in the biomedical field should be directly transferred to cell banking in the cultivated meat context. Biomedical testing and QC measures must always be more stringent, in particular for therapeutic applications in humans compared to a food context. The emerging CCP industry is currently still in the process of establishing together with regulators the relevant levels of stringency of QC and testing that ensure safety of products while enabling commercial viability of production processes.
Rapid advancements in stem cell research over the past 15 years have enabled medical applications in the Gene and Cell Therapy (GCT) sector, including regenerative medicine. In addition, stem cells and genetically engineered stem cells have become a highly valuable resource for fundamental research in biology and medicine. It is well acknowledged internationally that rigorous cell banking practices to preserve, trace and share, or commercially trade stem cells are a prerequisite for unlocking their scientific and medical potential. As stem cells are becoming an important element in the rapidly growing bioeconomy, public and private stem cell banks are currently established in many countries with the aim to promote intra- and trans-national access to ethically sourced, trackable and quality controlled stem cell lines (Huang et al., 2019; Knoppers & Isasi, 2010; Pamies, 2016).
Over the past 15 years a number of organisations and initiatives have set up and curate centres for stem cell repositories internationally, with the aim to ensure that high quality standards in cell banking practices are achieved and regulatory standards developed and met, in particular for medical applications and human derived stem cells. Examples are: the International Stem Cell Banking Initiative established in 2007, the Center for iPSC Cell Research and Application (CiRA) (Japan), the iPSC cell repository of the California Institute of Regenerative Medicine (CIRM), or the European Bank for induced Pluripotent Stem Cells (EBiSC), established in 2014, and the International Society for Stem Cell Research (ISSCR), among others (see table 1 in: (Kim et al., 2017).
Despite such international efforts, it is acknowledged that the scientific and procedural complexity of maintaining such cell banks is high, that more streamlining of methodologies and practices is still required, and that currently interoperability between large cell banks in terms of data formats, and laboratory processes is still low (Allsopp et al., 2019). However, international efforts to improve this situation, including data compatibility and accessibility, are underway (Chen et al., 2021). Ultimately, it is expected that international standards for food applications need to be established at the level of Codex Alimentarius, Comité Européen de Normalisation (CEN), and ISO and other standards-setting bodies.
When considering insights from the biomedical stem cell field and their applicability to the cultivated meat sector, it is important to note that the scientific complexity and laboratory methodology of maintaining stem cell quality and avoiding various contamination risks can be considered on a technical level as identical. However, the requirements for laboratory protocols and ingredients used vary in their molecular detail, depending on whether ASCs, PSCs, or immortal cell lines are used. It is therefore useful to distinguish between the procedural aspects of cell banking such as: what data are registered, what labelling systems are used, how traceability is ensured, and laboratory protocols with detailed constituents used for storage solutions and cell culture steps required during the cell bank setup process. It is likely that robust traceability of input cells will be the basis for possible future labelling requirements of CCPs, including for ingredients, origin authentication, and product quality descriptors amongst others.
It is generally assumed that Good Laboratory Practice (GLP), Good Cell Culture Practice (GCCP), Good Manufacturing Practice (GMP), and Standard Operating Procedures (SOP) principles must be adhered to when setting up stem cell banks. However, it needs to be considered that the underlying definitions, rules and regulatory frameworks impacting the details of QA processes demanded by regulators are still evolving (Pamies, 2016). Regarding GMP requirements for large scale production of stem cells it is acknowledged that GMP compliant processes must consider the industry/market for which the stem cell or stem cell product is intended. For example, when in a Biomanufacturing Facility Quality Management System relevant GMP needs to consider all parts of the production facility/process that directly come into contact with the stem cells produced. Some of the practical procedural aspects of good practice that need to be covered by such guidelines are shown in table 4 below (example: European Bank for induced Stem Cells EBiSC, (Steeg et al., 2021).
5.1. Example: recommended practices for production and banking of iPSCs for biomedical applications - overview
For use of stem cells in medical applications or when using stem cells obtained from humans, in many countries a set of legal requirements concerning ethical issues, consent, and data security and data handling among others apply. For the purposes of this report, we do not include reporting on these aspects.
5.1.1. Cell harvesting
Prior to cryopreservation the respective stem cell type needs to be grown to required quantities that allow proportionate aliquot sizes for efficient downstream use. Generally, cells are harvested as single cells or clumps at a confluency rate around 70%-80%. However, the optimal point for harvest can vary between different stem cell types. Ideally the cell population that is cryopreserved should be as homogeneous as possible with regard to passage number, proliferative behaviour and other parameters. For large scale harvesting it needs to be considered that the first expansion phase of stem cell banking is usually caried out using small scale laboratory methods. This means that usually cells from a number of separate culture flasks or plates need to be pooled prior to cell banking. Pooling of cells from many different culture vessels is a step that might increase the likelihood of pathogenic contamination or heterogeneity of the pooled cell population.
Currently, stem cell researchers widely use low-rate freezing dimethyl sulfoxide (DMSO)-based cryoprotectants (Steeg et al., 2021). However, a wide range of non-toxic sugar-based and food grade cryoprotectants exist and are successfully used in stem cell protocols (Kaushal et al., 2022; Mamo et al., 2024). For details of ingredients and protocols for cryopreservation of cells, a large body of textbook and academic literature exists, as well as numerous commercial sources (e.g.: (Capes-Davies & Freshney, 2021).
After freezing, master cell bank aliquots should be stored in -150˚ C freezers or liquid nitrogen dewars, ideally splitting each batch of master cell bank vials so they can be stored at separate locations to reduce risk of cell loss due to infrastructure failures. Storage temperature is important, and for example EBiSC recommends storing iPSCs only temporarily at -80˚ C (one of the most common temperatures used to freeze biological samples), and report that issues such as incorrect cell line identity, poor viability, and karyological abnormalities could be traced back to fluctuations in storage temperature (Steeg et al., 2021).
For upscaling cell banking approaches the following needs to be considered:
-
Whether cells should be stored in suspension, as single non-adhering cells, or as clumps.
-
The use of technical solutions, such as controlled rate coolers.
-
A good operational fit between storage formats at banking stage and required downstream use formats.
-
Does storage medium and format allow for a simple cell recovery (revival) protocol that is robust and reproductible each time a master cell bank vial is defrosted.
Several major QA control points are recommended by various stem cell associations to ensure cell quality and specific assays for each stage of the cell production and banking process are suggested. These are briefly summarised in the following sections.
5.1.2. Cell identity testing
Cell identity testing needs to be established and confirmed prior to cell banking with various DNA sequence analysis methods including Short Tandem Repeat (STR) analysis, and Whole Genome Sequencing (WGS). If genetically engineered cell types are used the integrity of the modified sequence elements needs to be verified. Robust protocols for data acquisition, recording, storage and sharing need to be established and must be part of QA processes.
5.1.3. Microbiological sterility
Microbiological sterility needs to be assured by applying an overall microbiological control strategy that covers the entirety of the cell production and banking processes.
This includes mandatory Mycoplasma testing using European, US, or Japanese pharmacopeia and otherwise other national pharmacopeia that specify test regimes and their limitations, specificity and sensitivity, as well as challenge or spiking tests as required. Such tests can be PCR, broth-, culture- or VERO incubation-based (using African green monkey cells as assay cells for readout for vectors that can also infect human cells).
Bacterial and fungal sterility must be confirmed using standard bacterial and fungal sterility tests, which could be broth or culture-based and must be recommended by pharmacopeia applicable in the jurisdiction where the work is carried out. In addition, other molecular methods may be used but are not sufficient alone. Endotoxin tests for the detection of gram-negative bacteria are recommended, but their limits of sensitivity and specificity need to be considered depending on test protocol and requirements in different jurisdictions.
Viral testing for all relevant species-specific adventitious agents needs to be performed (e.g. for human cells, Hepatitis B and C, and Human Immunodeficiency Virus (HIV); for other mammals their relevant viruses as established by veterinary regulations). In addition, in cases where media or cell culture ingredients from animal sources are used (e.g. serum, ECM molecules, and hormones among others) relevant adventitious viruses must be tested for. For products from bovine sources testing for prions is recommended.
5.1.4. Genetic fidelity and stability
Residual vector testing is required to exclude the possibility that genetic vectors used in the stem cell reprogramming process of iPSCs have integrated into the genome. Standardised methods for unequivocal detection of such genetic material in iPSCs are still under development and are currently being tested. Particular attention needs to be paid to a variety of in-house developed methods currently used by producers of iPSCs. Currently, it is recommended for high-quality research collections of iPSCs that an acceptance threshold of ≤ 1 plasmid copy per 100 cells should be observed and clearance to that level needs to be demonstrated in seed and master cell banks.
To technically execute such tests the following needs to be considered (established for human stem cells) as proposed by (Sullivan et al., 2018).
-
Testing platform: to increase accuracy and reduce the possibility of false-positive results, the use of quantitative PCR using sequence-specific DNA labelling chemistry, such as TaqmanTM , is recommended, rather than SYBRTM green that binds all dsDNA and might sometimes lead to false-positive results.
-
Two different regions, common to all plasmids used in the reprogramming system, should be chosen as specific targets, for example, OriP, EBNA, CAG sequences.
-
A standard curve should be prepared in a carrier of genomic DNA (gDNA) rather than water to accurately represent the test reactions. Ideally, gDNA from a well-characterized human pluripotent stem cell (hPSC) line (e.g., WA09) should be used.
-
Internal reference gDNA sequences should be incorporated to allow quantification, for example, RNaseP, hTERT. This is particularly important for the calculation of plasmid copies per cell.
-
Sensitivity should allow detection of ≤1 plasmid copy per 100 cells and standard curve(s) should be prepared to include samples at least 1 log below this level to demonstrate the limit of detection.
Karyotyping methods for assuring genotype of human pluripotent stem cells for research purposes have been standardised and been published previously (Andrews et al., 2015). Like in other cell types maintained in culture for a long time, genetic drift and the occurrence of culture-driven mutations has been demonstrated for iPSCs and human Embryonic Stem Cells (hESCs) (Tapia & Schöler, 2016). As it has been suggested that iPSCs might be more unstable than other stem cells, it is considered that genomic stability needs to be monitored more thoroughly in iPSCs, especially in cell production contexts with high doubling rates and large volumes (Peterson et al., 2011; Tapia & Schöler, 2016).
It is recommended that tests for karyotyping are carried out in a representative aliquot after culturing for 48-72 hours according to best practices. Twenty karyotypes (metaphase plates) should be analysed, which is a clinical standard internationally recognised by regulators in biomedical contexts supposed to give 95% certainty of diploidy in mammalian cells (Rooney, 2001). If abnormal karyotypes are found in the first test sample of 20 cells, then a second aliquot of 20 cells must be tested. If the second sample also shows abnormal karyotypes, the cell line must be considered compromised, and it is recommended to keep a record of the type of karyotypic changes observed.
Single nucleotide polymorphism (SNP) array testing has an approximately >50 times higher resolution than standard morphological karyotyping and has become standard practice as a test for diagnostic clinical cytogenetics (Miller et al., 2010; Sullivan et al., 2018).
Genetic and disease marker analysis
Whole genome sequencing (WGS) and other detailed genetic and disease marker analysis is currently not required by biomedical regulators for most biomedical stem cell banking. However, it is recommended to perform whole exome sequencing (WES) to the highest available depth and coverage. The occurrence of cancer-associated genetic changes has been of some concern among scientists as for example mutations in the TP53 gene (a tumour suppressor gene) have been reported to repeatedly occur in human iPSC cultures (Merkle et al., 2017). It is therefore recommended to screen WGS or Next-generation Sequencing (NGS) data collected from PSCs with existing mutation panel data repositories and such information be recorded. It is acknowledged that the actual risk posed by certain mutations depends on the application context and the final differentiation stage at which the differentiated cells are used.
5.1.5. Viability testing
To ensure consistency of cell quality and efficiency of cell culture processes, overall viability is usually tested to ensure for example in a clinical setting a consistent number of cells can be produced in a given time for patients. Standard cell viability tests are recommended to be carried out after 48 hours of culture after cell thawing and revival.
Doubling time assays are currently not mandatory but can provide to some degree assurance of genetic stability and overall health of the cell population. It is recommended to record the number of passages that cells have undergone and that cumulative doubling times are used as a measure wherever possible to document the proliferative history of cells (Sullivan et al., 2018).
5.1.6. Cell characterisation
Different types of stem cells (ESCs/PSCs, iPSCs) are expected to be in a stem cell state that reflects their specific “stemness”, depending on from which source they were derived. Hence, the risk of contamination with atypical and spontaneously differentiated cells needs to be controlled, particularly when setting up initial source (seed) cell banks. For example, for human PSCs cells well-established molecular marker panels are used to identify the stem cell state. Phenotyping via antibody-based immunodetection with a minimum of two markers of such a standard marker panel is mandatory. Immuno-phenotyping is usually carried out using Flow Cytometry, and currently used antibody markers for mandatory testing of hPSCs are OCT4, TRA-1-60, TRA-1-81, SSEA-3, SSEA-4, SOX2, and NANOG. A combination of one intracellular (e.g. OCT4, SOX2, NANOG) and one external or membrane marker (e.g. SSE-4, TRA-1-60) should be used. For research grade hPSCs characteristic marker profiles should be detected in >70% of cells. Immuno-histochemistry-based stem cell characterisation is currently not mandatory but when performed, again one intra-cellular and one membrane/extracellular marker should be used.
Relevant characterisation data, for input cells used to establish master (seed) cell banks for the production of research grade hPSCs have already been published earlier, but continue to evolve (Andrews et al., 2015).
5.1.7. Potency of stem cells
Stem cell potency is the defining property for cell differentiation into either a) any cell type of a given species (totipotent stem cells) or b) a more restricted range of cell types found in the body (pluripotent stem cells). Establishing and testing for the expected stem cell potency is therefore important for ensuring relevant biological properties, consistency and quality of the production process, and the final product that is made from stem cells. Inadequate molecular control of the specific “stemness” state is particularly detrimental for iPSCs as having cells of undefined phenotype in the starter cell population will contaminate the cell culture with cells of inappropriate cell function and phenotype. Phenotypic pluripotency assays are used to ensure potency of cells by using functional tests such as embryoid body formation and directed differentiation assays should be performed to demonstrate that all three embryonic germ layers (ectoderm, mesoderm, endoderm) can develop from a monolayer of stem cells. Any data that can demonstrate ectoderm/mesoderm/endoderm-specific marker expression is currently sufficient. Other methods such as molecular pluripotency assays based on gene expression data (e.g. mRNA arrays, RNA-seq) are now also commercially available, for example PluritestTM or hPSC ScoreCardTM (Bouma et al., 2017; Müller et al., 2011). Demonstration of pluripotency by such methods is recommended.
5.1.8. Testing for unintended DNA sequence change in genetically modified cells
A variety of gene modification and gene editing techniques such as TALENs or CRISPR-Cas based systems are now frequently used to introduce DNA sequence changes into stem cells and have become powerful tools in basic research and disease modelling approaches (Czerwińska et al., 2019). These more recent gene editing approaches are often described as less problematic in terms of unintended genomic effects, off-target effects, and random sequence altering effects. However, such effects can still occur frequently, depending on DNA sequence context and technical aspects such as design of guide RNAs (gRNAs), even though impact of these sequence changes on cell phenotypes are sometimes not readily detectable. In addition, on-target effects (OnTEs) and deletion events have been repeatedly reported (Weisheit et al., 2020). Hence, it is recommended to provide DNA sequencing information that shows that such unintended effects can be excluded by using techniques such as quantitative-genotyping PCR.
As a QC measure for genomic integrity and absence of unintended alterations following genetic engineering and prior to master cell banking some of the following tests are recommended in the academic literature, and relevant DNA sequence information should be provided (Steeg et al., 2021).
-
Genomic arrays such as G banding, SNP, or array Comparative Genomic Hybridisation assays (aCHG)
-
Target locus sequence data
-
Tests for intended impacts of genetic modification on gene expression levels (e.g. proof of over-expression, knock-out, or inducible expression)
-
Documentation of off-target effects using Next-generation Sequencing (NGS) or Whole Genome Sequencing (WGS) methods
-
Assessment of on-target effects using quantitative genotyping PCR
In summary, several important QC steps and their assays to ensure identity and quality of iPSCs are well established and documented in the academic literature. An overview of currently used assays, that are recommended (or are mandatory) for iPSC quality control measures throughout the process of establishing iPSC cell lines from primary tissues is shown in Figure 12 below (based on recommendations by EBiSC published in: (Steeg et al., 2021).
6. Summary of findings in reviewed literature
Production technology for cultivated meat is at an early stage of maturity despite a limited number of products already being approved by regulators for human or animal consumption.
Current limitations and bottlenecks within the methods and laboratory technologies used have become better understood over the past five years and substantial innovations at all stages of production can be expected in the coming decade. This includes innovations in cell engineering to produce cells better adapted to large scale production processes, animal source-free media and ingredients, bioreactor design, growth substrates and scaffolding materials and final product texturization and taste. In addition, all these process steps will require considerable upscaling to enable commercially viable production.
As no standardised methods or practices for the complete process sequence of cultivated meat production currently exist and various innovations are tested at pace, it is expected that high variability in practices and used input materials will continue to be a characteristic of cultivated meat production in the next 5-10 years.
However, awareness about the food safety aspects of cultivated meat production in the nascent sector appears high and is well discussed in the literature with information on compliance criteria for different jurisdictions available. In particular, risks and hazards originating during cell culture practices are reasonably well described within the cultivated meat production context. Increasing numbers of applications for product approvals to regulators - and related documents that are often publicly shared, contribute to an increasing understanding of what risks and hazards must be controlled by producers and which QC measures meet regulatory requirements. However, the scientific complexity to provide evidence for potential effects that process chemicals (cryoprotectants, novel media components, cell protectants, biologically active molecules including growth factors and hormones) may have on humans is high and needs to be assessed on a detailed case-by-case basis.
As the foundational production methodologies originate in biomedical laboratories, the regulatory demands for biomedical applications are well understood. What is less clear currently is which parts of the production process need to be carried out under stricter regulatory frameworks and which parts might be somewhat relaxed within a food context compared to biomedical production. One future scenario might also be that only some specific production stages might be regulated by very similar regulatory frameworks. For example, for possible future GMO, authenticity, or QC labelling requirements initial production stages might need to be more rigorously documented and specific QC processes carried out for which currently no “industry standard” has yet emerged. This might be of particular relevance for early stages such as cell banking as it is the foundational step that defines the quality of the starting material (cells) of the production process. It is also the cell characterisation that defines the banked cell that would be used to assess the quality of the end product in terms of consistency of the production process.
Explicit information on cell banking practices within the cultivated meat context is currently very limited and can only be inferred indirectly from the occasional individual publication on technological advances. It appears that cell banking practices are considered “standard” methodology by cultivated meat producers, although the specifics and details of cell banking protocols can vary considerably depending on the cell type (different types of stem cells) or the species of origin.
Moreover, the literature on cultivated meat is often not clear on the stage at which a cell bank is considered the master cell bank and whether several additional cell banking steps are carried out for different purposes further downstream and what their specifics are. Hence, clearer definitions for the types of cell banking carried out specifically in the cultivated meat context need to be developed to support regulatory decision making on cell banking QC requirements.
It is also anticipated that cell banking practices will need to be scaled up to unprecedented scales should production volumes reach industrial scale. As the required procedural and continuous testing requirements would also reach unprecedented complexity. With associated high costs, cell banking and provision of relevant genetically modified cells might become an industry in its own right, supplying cultivated meat producers at large scales in the future.
Cell banking practices and regulatory requirements for stem cells used for biomedical applications are reasonably well documented and understood, despite having evolved at larger scale only over the past 15 years. However, international efforts are still ongoing to streamline and standardise procedural aspects (data collection, data formats, legal data requirements among others) as well as best laboratory practices for stem cell banking with regard to QC points and required mandatory tests to ensure cell consistency and quality. Best practices in the biomedical field should be actively investigated, specifically in regard to which aspects of cultivated meat production they would be most relevant for and transferable, with potential regulatory requirements in mind.
While the stringency of some QC processes currently used within a biomedical setting may be relaxed compared to what would be expected in a food context, the transfer of good cell banking practices from the biomedical sector to the food context of cultivated meat might be desirable. Safety aspects relating to the cells themselves (e.g. phenotype, gene expression, unintended allergen production) could still apply, as our understanding of what cellular changes should be deemed permittable and what changes might have an impact on human health in the cultivated meat context, is at present unknown.
7. Expert elicitation workshop
An expert elicitation workshop was conducted to capture latest information and insights on cell banking practices in cultivated meat production, given the novelty of the technology, its rapid evolution over the past five years, and the current lack of academic publications on the subject. Experts were selected from cultivated meat producers and companies supplying cells, production process ingredients, and laboratory technology to them. In addition, CCP experts from academia including from adjacent fields such as cell banking for biomedical purposes as well as experts in food safety, regulatory aspects, and food production processes were contacted. Care was taken to ensure that a majority of participants have experience with cell culture practices and a good understanding of the regulatory aspects of cell culture-derived products and services either in a food or biomedical context.
7.1. Expert selection and pre-workshop questionnaire
Expert search was carried out globally using open-source information, ensuring a good international spread of academic and commercial expertise. A range of different cultivated meat producers were contacted representing different product categories in terms of origin species of cells including cattle, chicken, and fish and seafood products. In addition, care was taken to include companies covering a range of technological and commercial maturity, and experts at different levels of seniority. One hundred and forty-five relevant experts were initially contacted and requested to participate in the workshop and to complete a pre-workshop questionnaire/survey. The questionnaire was intended to better gauge the area and depth of expertise of potential workshop participants and to get an initial understanding of the specific potential food safety hazards along the production process, whether there are specific critical steps/process stages that needed to be considered, and what role regulation might play for this emerging food production technology. Of the 145 experts contacted, 39 returned a completed questionnaire, and 19 took part in the online workshop. For details on expert characteristics and answers to technical questions submitted, see appendix B.
7.2. Workshop format and discussion themes
The workshop was conducted online using the videoconferencing platform zoom, to help with ensuring a good spread of UK and international participants and time efficiency. The workshop opened with introductions, a brief overview of pre-workshop questionnaire outcomes, and research conducted so far. Three main themes with a top-level question and sub-questions were discussed by participants in three rounds of breakout room discussions. Discussions were held in four separate breakout rooms with approximately 5 participants each and lasted approximately 25 minutes; each group was supported by a facilitator from the Camrosh team and the two advising experts of the project. After each discussion round, facilitators reported back to all participants on the insights and discussion points in the individual breakout rooms. A short general discussion was then held between all participants after each round of break out room discussion. After the last round of breakout room discussion and report-back, a final overall discussion was held followed by a brief online poll on five specific questions relevant to the workshop themes.
The three main workshop themes were aimed at gaining a deeper understanding of current practices and workflows, potential hazards and mitigation approaches at early process steps including cell banking, the role of genetic stability of cells used in production, and regulatory aspects that might be relevant to cell banking practices. The following three specific themes were discussed.
Theme one: early process steps, sequence, hazards and control points
To stimulate discussion, participants were shown a generic flow diagram depicting an overview of the most common early process stages in cultivated meat production from cell sourcing to master cell banking and thawing of vials from a working cell bank for initiating a production run. This diagram was intended to act as a primer for discussing currently performed process steps, their sequence and possible control points for testing requirements along the process. The diagram was based on information from a proposed HACCP document provided by a commercial supplier of cell lines for cultivated meat production and included process steps for cell line modification. For details of the diagram, see Appendix C.
The overarching question to be discussed (including sub-questions) was the following: “Does this flow diagram reflect the actual current cell banking practices and is it suitable for food applications?”
The following prompting sub-questions were provided with the primary question:
The questions to consider are:
- Does the flow diagram reflect the actual current cell banking practices and is it suitable for food applications?
- Is there anything from microbial banking practices which may be useful/relevant?
- Are there any steps that are not relevant to food production and mainly serve pharmaceutical and clinical applications?
- Which steps are critical for downstream processing steps, irrespective of whether it is for a food application or not
- Are there any food specific requirements/steps missing that should be added, if so, what are they?
- Chemicals (e.g. additives, veterinary residues etc) and growth factors/hormones; are there any which are a clear hazard or potential risk (due to concentration added or nature of the chemical); is the information required to carry out appropriate risk assessments available, if not how can it be obtained, and if it is available, have appropriate risk assessments been carried out?
- How can identified risks be mitigated?
- What are the key assays (e.g. toxicological/analytical, microbial and chemical) in early cell banking stages to ensure safety of the final product?
- Other points to consider?
Theme two - the role of genetic drift of cells used for cultivated meat production
This theme was selected because the evidence base in the academic literature, and the scientific study of potential risks posed to humans by genetic drift in CCPs, is currently very limited. The overarching question discussed was the following:
“What is the likelihood that genetic drift occurs or is induced in the early cell banking and propagation steps and how can it be controlled?”
The following prompting sub-questions were provided with the primary question:
What is the appropriate end point to aim for?
- How often and with which assays should genetic drift be monitored during production?
- When is genetic drift going to have safety implications, is there enough evidence to answer this question?
- Is there a specific point along the production process where genetic instability is more likely to be a hazard and become a risk?
- How can producers demonstrate that genetic drift is sufficiently controlled?
- What tests should be conducted?
- What data should be provided?
- Which hazards may be introduced by genetic drift? Which are most relevant for food products e.g. novel allergens due to mutations, novel metabolite residues, emerging cancer cells? What will be needed to horizon scan for or anticipate potential hazards, how can they be risk assessed and what mitigations might be applied?
Theme three: - regulatory context and the possible role regulation might play for cultivated meat production processes
The overarching question asked was:
“How could specific regulatory requirements for cell banking practices help with establishing best practices in the emerging industry, build consumer confidence and support further necessary innovation?”
The following prompting sub-questions were provided with the primary question:
What are key regulatory requirements that should be considered by the FSA?
- Standardisation of cell banking practices vs necessity for IP generation
- What is considered IP in the cultured meat "cell banking" process?
- What is the impact of any standardisation for Cell Cultured Product (CCP) producers?
- What guidelines can be implemented in the safe production of CCPs? (e.g. acceptable additives to the culture media)?
- Other points to consider?
Given the conversational nature and the time constraints of the workshop, not all sub-questions were answered systematically.
7.3. Workshop outcomes: insights and expert opinions
In this section salient insights and opinions held by experts on the three themes introduced above are presented. Main insights are considered points of wider agreement on specific thematic questions that could not be retrieved by literature research alone or include information that would be difficult to assess with regard to relevance and level of application for current cell banking practices, without expert input. Within each thematic area, the order in which major insights are presented in the following sections does not indicate perceived importance of the individual point, or degree of agreement amongst participants. We would also like to point out that the opinions reported below only represent the views of experts as they contributed to the workshop and are not meant to represent positions and opinions or policy considerations of the FSA.
7.3.1. Main insights on early process workflow, hazards, and current practices of cell banking (theme 1)
Presented with a generic workflow diagram of early production process steps participants agreed on the overall validity and broad applicability of the shown steps; however, they discussed and highlighted the following points/issues in more detail.
Early laboratory steps and practices prior to cell banking vary considerably between producers depending on source cell type and species used
Proposing a rigid process workflow for early production steps is seen as unrealistic and would not be considered helpful to guide future developments. Different approaches and variations in early process stages exist mainly for two reasons:
Firstly, the animal species used for cultivated meat production are mostly non-model organism species, which generally have a shorter history of scientific research, fewer standardised practices, and less scientific data available (such as genomics data and species-specific assays and tests). This is opposed to cells from so called model-organisms (e.g. mice, frogs, fruit flies, nematodes) and some human cell lines that have been intensively studied for over 100 years. Cultivated meat producers have only been optimising cell culture practices for meat production for little over a decade, with least scientific data available for fish and seafood cells.
Secondly, different developmental stages of cell types and various tissue sources are used as the starting point for production processes prior to banking. Cells might be sourced from biopsies from a live animal or tissue samples from a cadaver to isolate adult tissue stem cells, from embryos and eggs, or derived via induced pluripotent stem cell (iPSC) routes. Starting cells can also first be immortalised and modified or purchased from a cell supplier that has already performed all the initial isolation and modification steps prior to banking. Hence, these different cell origins dictate the variable and different practices that these cells require.
One agreed source of differences in early process steps is biopsy/tissue material: storage conditions (mostly cold, not frozen), type of media, and critical time to processing can all vary considerably between species.
Given this current heterogeneity in early process steps prior to and including cell banking, it was suggested that any “generic” workflow diagram would need to be constructed in a more modular fashion, such that different, species-specific process steps and relevant testing might be performed at different time points in the workflow. However, the overall process should achieve similar standards at the cell banking stage regarding management of hazards and food safety.
Origin and traceability of cells throughout the process is considered essential – although currently no standardised approaches exist
There was unanimous agreement among experts that standardised practices and methods must be established to ensure traceability of cells from source to product, ideally using digital documentation systems. It was pointed out that the generic workflow diagram presented to participants did not explicitly show tracing steps/methods. Different suggestions how this could be achieved were made, including: making use of already existing veterinary documentation systems (for biopsy or tissue material from animals already in the food chain), documenting health history and veterinary drug administration of donor animals, establishing a structured secure “passport” system for cells, blockchain and other digital ledger technologies, and use of genetic and intracellular molecular markers. It was also suggested that traceability measures must also include existing GMP, GLP, and GCCP, guidelines for secure labelling and documentation of stored cells and related chain of custody documentation, as is well described for biomedical cell banking.
However, it was also pointed out that cell origin traceability is currently not established in the fish and sea food segment. This would be difficult to do as they are often sourced from purchased wild-cought specimens. In addition, there is currently insufficient genomic reference data available for fish and sea food organisms, making it difficult to characterise cells. Moreover, participants pointed out that cell tracing requirements should also not be based on unreasonably elaborate/time-consuming/expensive procedures (e.g. using specific genetic markers) and should be somewhat more relaxed in a food context compared to biomedical cell banking to keep costs and time requirements of tracing systems manageable.
It was suggested to use and refer to existing food industry documentation systems, including veterinary documentation, when establishing cell traceability frameworks. Traceability documentation systems are well established in the food sector, are underpinned by existing food law, and clear guidance exists in the UK and EU.
Testing for bio-pathogens and cell characterisation are performed predominantly at the cell banking stage
Given the great heterogeneity of early process steps prior to banking, depending on cell origin and cell types used, there was agreement among participants that most of the hazard testing and cell characterisation should be performed mainly at the cell banking stage. Time and cost constraints and workflow complexity would make testing between every initial step prior to banking impractical. However, according to workshop participants, some companies have established a parallel workflow for hazard testing while setting up the master cell bank. A second round of hazard testing and cell characterisation testing might be carried out at thawing of a working cell bank vial before it enters a production run. Several participants pointed out that on biopsy and tissue material basic viral, microbial and mycoplasma testing should be performed on the collected tissue sample as early as possible.
Participants agreed that testing for bio-pathogens such as viruses, bacteria, fungi, and prions is crucial and needs to be prioritised before cell banking with utmost attention in terms of test sensitivity and specificity. This also applies for testing commercially available media components of animal origin, and for any cells purchased from commercial suppliers.
Most participants agreed that cell characterisation at overall phenotype level would be sufficient during early process steps including during initial cell expansion/proliferation rounds. It was also suggested repeatedly that full, detailed cell characterisation using a range of techniques (e.g. karyotyping, WGS, transcriptomics, testing for specific short tandem repeats (STR), among others) should be performed at the stage before or after vials are frozen to establish the master cell bank. For a reasonable approach to ensuring phenotypic cell identity, most participants agreed that cell characterisation assays should be performed at the working cell bank stage, before entering a production run, and at the end of the downstream processing prior to final product formulation (mincing, layering, texturizing, combining with other cell types). However, it was also pointed out that most products would contain several different cell types, and that cell characterisation testing would need to be based on robust and simple techniques, such as for example short tandem repeat (STR) testing, karyotyping, or morphology assessment.
It was also pointed out that intermediary, smaller cell banking/storage of cells already in a downstream production process might help with QC processes, although this could substantially increase workflow complexity and costs.
The potential risk of carrying over chemicals and bioactive molecules from cell banking vials into downstream processing was perceived by most participants as small. They argued that most chemicals and bioactive molecules in a single cell bank vial would be diluted out substantially in subsequent process steps over many days and weeks of production. However, with respect to downstream processing steps (including cell differentiation, texturizing, and final product formulation steps), there is limited knowledge of what levels of residual molecules from downstream steps would be permissible, and what specific molecules would need special monitoring. It was argued by participants that final processing of cultivated meat products, such as freezing, frying, or cooking might inactivate or degrade most of the added molecules required for cell culture and processing. However, the concern would be raw or semi-raw consumption of the product, for example in the fish and seafood sector in the form of sushi or sashimi, or with other meats in rare and medium-rare preparations. Hence, participants agreed that more guidance on what residue testing needs to be mandatory, and for what molecules/chemicals.
For a table of chemicals and biological molecules considered by consulted experts as frequently used and important in early production steps, see table 12 in Appendix B. For a table of compounds considered by consulted experts as potentially of concern, see table 13 in appendix B. Information in tables was obtained through the pre-workshop questionnaire.
Several participants pointed out that using food-grade ingredients routinely for master cell banking steps might simplify meeting regulatory requirements. However, it was also argued that with rapidly evolving media compositions tailored to specific species, or in efforts to replace components of animal origin, many novel ingredients might be used with no prior history of use in a food context. This might require that such components would need to become first classified as food-grade in separate approval processes.
Master Cell Bank (MCB) and Working Cell Bank (WCB) definitions
Since it appeared that, in the academic literature, definitions of what is considered a MCB and what a WCB can vary with respect to their overall position within a given workflow, several participants agreed on the following core defining characteristics of MCBs and WCBs.
A MCB serves as the primary source of all cells for production, typically contains at least >100 vials, and cells are intended for long-term use over many years. This includes use for research and modification purposes. Cells in a MCB are expected to be thoroughly characterised and have their origin well documented. A MCB can contain various cell types in terms of differentiation stage such as primary adult stem cells, PSCs, immortalised cells, and embryonic stem cells amongst others.
A WCB is derived from one or more vials of the MCB. Cells from a WCB vial are expanded to desired quantities for day-to-day production. Downstream process steps, in which cells from the WCB are used also require ongoing QC and testing steps to ensure phenotypic stability and safety of WCB cells over the entire time span of the production process.
7.3.2. Genetic drift: likelihood, testing and control of genetic drift in early process steps and cell banking (theme 2)
As genetic drift of cells over time is a well-known phenomenon in whole organisms and in cell culture, participants were asked to discuss its role in cell banking and QC of early production processes in a cultivated meat context.
Several factors are expected to impact rates/ocurrance of genetic drift
Scientific evidence that measures the specific role of these factors in current processes is currently lacking. Participants pointed out that the rate of genetic drift can be impacted by many intracellular and environmental/external parameters in a highly complex manner with the following two factors likely being the most important for impacting the rate of drift in cultivated meat production processes.
-
Cell type and differentiation stage: Participants pointed out repeatedly, that while genetic drift is expected to happen, the rate at which it occurs is likely to be cell type dependent. There is also some evidence that less differentiated cells such as pluripotent stem cells can drift faster than more differentiated cells such as resident tissue myoblasts, satellite cells, or mesenchymal stroma cells (Jaime-Rodríguez et al., 2023). However, it also needs to be considered that cultivated meat products usually consist of several cell types, and monitoring different genetic change rates consistently across production stages does not appear to be feasible.
-
Production processes: As cultivated meat production attempts to achieve unprecedented scale of cell culture with very large numbers of cells involved, participants pointed out that these upscaled processes need to be well controlled and investigated for rates of drift. Currently there are no reference data for what would be considered an expected or acceptable rate of genetic change for specific cell types and production processes. Also, data on whether certain media components, or specific cell culture conditions would increase rates of genetic drift are currently lacking. It was expected by participants that different production processes might lead to different rates of genetic change.
Currently, there is a lack of reference data on acceptable/normal rates of drift
Participants argued that natural rates of genetic drift in food production livestock (when cattle, pig, or chicken cells are used) might be used as reference information to establish baseline levels of genetic drift. However, the scientific complexity and required efforts involved in selecting/agreeing on specific genomic regions to test for variation, or even defining baseline “normal” rates for livestock cells is considerable. This would be even more so for fish and seafood species as even less information about cells of these species is available.
It was pointed out that the main issue would be establishing what genetic alterations might present a risk for humans in terms of affected protein function (or quantity). A change in allergenicity of certain abundant proteins in the final product was suggested as a conceivable risk that might arise through genetic drift although so far, no scientific evidence for such cases is available.
The causal links between genetic drift and potential risks for humans are currently not well understood
Participants agreed that currently there is no scientific evidence for a specific food safety risk to humans that might be introduced through genetic drift. Some speculative possibilities of such risks were proposed by participants such as novel genetic mutations/alterations in the genes of certain abundant proteins giving rise to novel allergens, novel metabolite residues of unknown effects, and cancerogenic transformation of cells. However, participants argued that there is to date no evidence that consuming products containing cancerous cells would pose a risk to humans as transfer of genetic elements into the human genome was considered highly unlikely. It was also considered that communicating this issue to consumers and the public might be problematic, should labelling of products require to indicate that a certain proportion of cells in a product might be cancerous cell types. There was overall agreement among participants that correlating specific genetic change to risk factors for humans in consumed cells would be highly complex scientifically, and considerable efforts in basic scientific research would need to be undertaken to provide baseline data for cells currently used in cultivated meat production.
It was repeatedly argued by participants that cells that would undergo substantial genetic change during the production process are most likely not viable or would disrupt the production process to an extent that they will be discarded based on issues related to production process efficiency.
Genetic drift and speculative, potential allergen risks
Although no direct scientific evidence exists to date, genetic drift might conceivably lead to changes in allergenicity of proteins abundant in cultivated meat products. Two examples of such proteins present in muscle were given by workshop participants, namely, tropomyosin (a crustacean allergen) and parvalbumin (fish allergen). While muscle tissues of most species contain these proteins it is currently unknown which mutations might generate more allergenic variants of these proteins and in which species.
In general, allergenicity of cultivated meat products might need consideration in the context of known rare forms of meat allergies against bovine serum albumin, mostly in children. The only other currently well documented allergen risk posed by meat products is the alpha-gal syndrome (AGS) or Mammalian Meat Allergy (MMA). AGS is caused by an immune cross-reaction to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal) contained in red meat (Macdougall et al., 2022). The immune response in humans is triggered due to priming of the human immune system after tick bites through which this carbohydrate is transferred via blood from other species to humans. AGS is the first known allergy that can lead to delayed anaphylaxis and the only food allergy against a carbohydrate rather than a protein. This may imply that cultivated meat needs to be treated with regard to allergen concerns like conventional meat.
Testing for genetic drift/stability
Given the scientific complexity and mostly unclear causal connection between specific DNA sequence changes and cellular phenotype and metabolism (most genetic changes are “neutral”), participants agreed that testing for genetic stability needs to be carried out in a pragmatic fashion mainly to ensure consistency of production processes. Routine testing of overall phenotype (cell morphology and specific molecular markers) should be carried out at the cell banking stage as part of initial cell characterisation. It was suggested that a realistic approach to genetic stability testing would be to test cells twice, at WCB vial thaw, and at late downstream processing steps before final product assembly and packaging. For routine QC testing, less elaborate methods such as overall morphological phenotype assessments (microscopy), low-resolution gene expression profiling, or karyotyping (although for some species this is still difficult to do), and possibly STR testing were suggested. For the production process, “phenotypic stability” (ability to form muscle fibres, adhere to substrate, maintain expected division rates among others) was considered to be more important than specific measurements of genetic stability. Hence, it was suggested that for future testing requirements, the focus might need to shift from defining genetic stability at the DNA sequence level, to definitions of “phenotypic stability” as being a valid proxy for genetic stability. In-depth testing with methods such as WGS, partial sequencing at lower resolution, or flow cytometry, were considered as too time consuming and expensive for establishing genetic stability when monitoring day-to-day production.
However, participants pointed out that a yet unspecified level of cell line stability must be demonstrated by producers to regulators to be able to fulfil the specifications to which the cells in question were approved. If cells that were substantially different from the ones that were demonstrated to regulators would be used in products, these products would be illegal. However, such a situation might according to participants arise frequently, given that the industry is in its early stages and speed of R&D innovation moves faster than regulatory approval processes. Currently there is no guidance relating to degrees of genetic change (deliberate, or through drift) that would require re-approval of cell types.
7.3.3. Regulatory considerations in cultivated meat production processes (theme 3)
As workshop participants have already indicated in the pre-workshop questionnaire (see Appendix B), there was agreement that more specific guidelines from regulators would be helpful for certain process stages. It was also pointed out that different jurisdictions can have considerably different regulatory specifications and information available with regards to different cell types (e.g. in the US substantial guidance on the process requirements of fish and seafood cells exist, while in other jurisdictions such guidance is limited). In the following we present major areas in which participants agreed that more specific guidance would be helpful for the emerging cultivated meat sector, while at the same time it was important that regulation is not overly prescriptive as the field is still dynamically evolving.
Areas that would benefit from clearer regulatory guidelines, suggested by participants
The following areas considered by workshop participants as in need for clearer regulatory guidance reflect solely the opinion of invited workshop participants, and are not indicative of opinion or policy intent of the FSA.
Cell origin documentation and traceability requirements were mentioned repeatedly by participants as an area that should be defined more clearly by regulators. Such guidance might for example include information on how best to integrate and use already existing documentation and tracing systems used for food production and veterinary purposes in livestock in a more standardised manner in the cultivated meat context.
Guidance on types and frequency of tests required to characterise cell types should take a food context into account and might not require the same depth of testing as biomedical cell banking. Given that most cultivated meat products contain several cell types, guidance on what level, or proportion of deviation from intended phenotype would be acceptable in final products might be helpful. It was suggested that guidelines for cell characterisation testing (to demonstrate identity and genetic stability of cell lines) should remain at overall phenotype level and more elaborate genomic testing only be used at initial master cell banking stage. Cell characterisation testing is also relevant for authenticity testing of final products. However, what tests would be most appropriate given the cultivated meat context is currently unclear (and to what extent authenticity testing methods for conventional meat are applicable). For example, STR testing was recommended as it is efficient in human cell banking, is a robust and cheap method, and GMP test providers are well established. However, the Official Food Control Laboratories (Public Analysts in the UK) would need guidance on how to set up and carry out testing requirements for the cultivated meat context.
Defining quality standards and hazards of process/media ingredients. These should ensure food safety and therefore a practical approach would be to aim for food-grade ingredient requirements rather than pharmaceutical grade ingredient requirements. Different jurisdictions have developed different approaches for acceptable ingredients, such as white lists (Singapore), or Generally Recognised As Safe (GRAS) lists compiled by the FDA for the food and beverages industry in the US. However, GRAS does not indicate automatically premarket review and approval by FDA of the used ingredient. In Great Britain (England, Wales and Scotland) and the EU more comprehensive risk and safety assessments are required.
Another contentious issue regarding GRAS lists pointed out by participants was the lack of a definition of safe levels of ingredients, and the fact that the understanding of safe may change, based on new scientific insights. The example of sugar was given, that was allowed in many countries to be used “safely” by food manufacturers at deliberate amounts, until the long-term health implications of high sugar consumption were clearly demonstrated by scientific evidence, changing the safety perception of sugar levels (and leading to taxation of sugar use by the food industry in some countries such as the UK).
Framework guidance on permissible residual levels of media and process ingredients and related testing requirements. These ingredients might be potential allergens, toxins, or be bioactive molecules (hormones, growth factors, small molecule inhibitors) when remaining in the final product. Currently, many bioactive molecules are not well documented for the cultivated meat sector, and generally many currently used media components have no prior history of being used in food production at scale.
Guidance for bio-pathogen testing and risk assessments might need to consider that some pathogens are species specific. Certain pathogens specific for pig cells were mentioned as an example.
Clearer definitions and requirements for MCBs and WCBs were considered useful by many participants for promoting more standardised approaches to cell banking. While standardisation efforts have been underway for decades in biomedical cell banking, it was argued by some participants that the great variety of used cell types and species might make it difficult to introduce too strict requirements for cell banking processes.
Clearer guidelines on biopsy derived cells from livestock animals were considered useful, in particular as in the UK there is currently no regulatory approved route that cells harvested from a live animal can enter the food chain.
Guidance on statistical sampling sizes when testing for different hazards in MCB and WCB vials. As an example, to prove sterility of human cells for biomedical use in the US, 1% of vials of the cell bank need to be sterile, and international guidance on sterility testing and harmonisation efforts across jurisdictions exist. However, at present, no such sampling criteria for sterility testing are explicitly defined by regulators for cell banking in the cultivated meat sector.
Guidance on sample size, testing frequency and assay types for standard QC processes along the production workflow downstream of cell banking was considered important by participants. Currently the level of scientific depth and frequency of testing requirements in relation to the scale of the production process is not defined in quantitative terms. For example, how many samples/cells need to be tested over what time scale and with which assays to reflect the dimensions of the production operation.
IP concerns and standardisation efforts
There was broad agreement among participants that cell banking practices as such might not be an IP concern for cultivated meat producers since mostly prior art approaches are used. It was suggested that IP mostly resides in specific media compositions, in formulations that use novel ingredients to replace ingredients of animal origin, and compounds that increase proliferation and differentiation efficiency as well as cell survival rates. Other areas of innovation mentioned by participants that might be worth protecting with IP rights were cell isolation and immortalisation methods, genetic modifications that improve performance of cells, enzymatic treatments during some process steps, bioreactor design, and biopsy acquisition and transportation methods among others.
While companies are expected to provide the required details to regulators in food product approval processes, they do not want to share information with competitors. Participants therefore suggested that a platform held by a trusted institution, where cultivated meat producers might be able to share information and best practices anonymously with regulators and industry bodies might be helpful to establish best practices more quickly within this emerging industry. The FSA is currently in the process of developing a system in which applicants can share information confidentially.
Participants agreed that overall, standardisation of some process steps would be useful despite the great variety of cell types currently used. However, standardisation efforts should not be driven by overly narrow regulatory requirements, but rather industry led risk-based testing approaches.
7.4. Post-workshop poll on 5 questions of relevance to the workshop themes
After conclusion of workshop discussions participants took part in an online poll answering the following questions.
“Would potentially harmful chemicals (cryoprotectants/media components) and bioactive molecules (hormones/growth factors) from cell bank vials be diluted sufficiently in downstream steps to mitigate their risk when consumed?”
As participants were discussing various hazards throughout the workshop, we wanted to capture whether the clearly defined context of the workshop themes would allow identification of main hazards that can pose risks to humans. The following question was asked immediately after the workshop.
“What residual components from the cell banking process are posing the biggest risk for humans? Select only one!”
To gain insights on currently used workflows in early process steps and cell banking, participants were shown a “generic” workflow diagram (See Appendix C). To assess the degree to which this diagram was reflecting actual current practices the following question was asked immediately after conclusion of the workshop.
“Does the flow chart discussed in first break out room session represent an appropriate model for cell line control in CCP production?”
From discussions in the workshop groups, this almost equal spread of opinion can be explained by the fact that some participants thought it would not cover enough detail although it was considered overall representative. Others suggested that it might be too rigid to reflect different requirements for different cell types from different species. Hence, the following follow-up question was posed.
“If your answer to above question is NO, what are the challenges?”
As genetic drift was discussed in the workshop by all workshop participants with a view to possible impact on consumers and potential ways of testing for genetic drift in a commercially feasible manner, the following question was asked.
“What would be a proportional measure for producers to demonstrate that genetic drift does not pose any risk to consumers (multiple choices)?”
7.5. Summary of workshop outcomes
This summary reflects the opinions of workshop participants, and not the opinion or policy intent of the FSA.
Cell banking practices/early process steps
Currently there is a high degree of diversity in early process workflows and practices for cell banking in cultivated meat production. Due to the diversity of cell sources, developmental stages, and species used in the industry, adhering to a rigid and universally prescribed workflow would neither be feasible nor beneficial for regulatory guidelines and development of industry standards. A modular approach would be recommended allowing for the incorporation of species-specific process steps while ensuring standardised final food safety and hazard management standards at the cell isolation and cell banking stages.
Another key issue to consider is a lack of standardised guidelines and practices for cell origin traceability. Some of the discussed solutions were:
-
Use of currently available veterinary documentation information and systems
-
Application of blockchain technologies or other safe digital methods to trace cell lines and cell banks to the source of cell origin (e.g. individual animal that was the source of biopsy
-
Use of robust and simple molecular markers for cell bank and final product QC
However, the industry still needs to establish efficient and cost-effective tracing methods for standardised large-scale testing that might be used more widely to harmonise cell origin traceability practices. It was pointed out that this might be particularly challenging for the sea food sector as mostly cells are sourced from wild catch. Hence adaptation of existing food industry documentation systems with consideration for novel regulatory demands for use of these cells in the cultivated meat sector is advisable according to workshop participants. Standardising requirements for documentation of cell origin was suggested to enhance transparency and likely to facilitate regulatory approval processes.
There was strong consensus on performing the most rigorous hazard testing and cell characterisation at the cell banking stage. The need for streamlining testing approaches was emphasised for enabling the development of more standardised quality control and compliance processes. It was recommended by participants to:
-
carry out the most rigorous bio pathogen testing early in the process
-
perform the most comprehensive cell characterisation at the master cell banking stage
-
prioritise safety while maintaining workflow efficiency.
However, further research is required to determine the extent of acceptable residual molecule monitoring necessary for downstream processing steps, particularly when final products are consumed in raw or semi-raw forms.
Although there is a common understanding of quality standards for media and process ingredients, it was emphasised that food grade testing requirements of ingredients should be applied, rather than pharmaceutical grade testing requirements. However, challenges are expected due to different levels of harmonisation (or an absence thereof) between jurisdictions.
Regulatory considerations for the use of novel ingredients in culture media and various parts of the production process are required as new ingredients without a history of use in the food industry are used in the development of advanced culture media for optimisation of production and yield as well as replacement of currently used animal derived ingredients.
Development of guidance on permissible residual level of media components particularly due to potential presence of bioactive molecules such as hormones and growth factors in final products was also deemed necessary by workshop participants.
Although some definitions for Master Cell Bank (MCB) and Working Cell Bank (WCB) are already available through cell banking literature in the biomedical field, there is a need to assess suitability of these definitions for the context of cultivated meat production processes. If emerging, adapted definitions of MCB/WCB might need to inform regulatory guidance on cell banking.
Leveraging food-grade ingredients for master cell banking processes was suggested to be effective for achieving faster regulatory approvals. However, considerations of novel ingredients in culture media currently under development will be essential as there is still a lack of understanding of their risk profiles when entering the food chain.
Genetic drift
Although genetic drift is reasonably well researched in biological systems, its implications for cultivated meat industry still requires further investigation to establish reference data, risk assessment approaches, and more scientific evidence of potential molecular mechanisms. Genetic drift rates might be influenced by multiple factors such as:
-
cell type
-
differentiation stage
-
production process and conditions.
Furthermore, cultivated meat production typically involves multiple cell types which adds complexity to tracking genetic drift and cell line consistency across the whole process flow. This is complicated further by a current lack of understanding of the impact of specific media and culture conditions on molecular mechanisms of genetic drift. Therefore, further systematic investigations into how production environments, including cell banking conditions influence cellular genetic stability is required.
On a more practical level, the absence of reference data on acceptable or normal rates of genetic variation for cultivated meat cells of various species poses a considerable challenge. Potentially livestock genetic drift data could serve as a benchmark. However, defining the baseline for genomic stability for different cell types still remains a scientific challenge; assessing the potential risks of genetic drift without clear reference data will be difficult, particularly in terms of food safety.
There are also other concerns such as potential changes in allergenicity of proteins and metabolic alterations which may have food safety implications, although currently there is no direct evidence linking genetic drift to allergenicity risks. This may become a more pronounced challenge as and when more products find their way to market and through wider consumption allergic reactions might become evident that would then require scientific investigation.
A pragmatic approach for testing genetic drift was advised, prioritising production consistency rather than extensive genetic characterisation. Phenotypic assessments were deemed more feasible for day-to-day cell monitoring as they are cost effective and proven sufficient for product consistency. By establishing definitions of acceptable phenotypic variation in cells of the final product, and a focus on overall phenotype level testing rather than exhaustive genomic analysis at every stage, robust testing standards that are feasible for producers might be developed.
Regulatory context
Experts consulted for this study mostly agreed that clearer guidance from regulatory bodies across multiple process stages might be useful in shaping the emerging sector. There was a unanimous agreement that regulatory requirements to ensure consistency, traceability, and safety would be perceived as supportive to the industry if innovation and scale-up of the industry are not restricted.
The generally slow process of regulatory approval when a cell line is submitted to the regulator, coupled with still ongoing in-parallel research and development on the cell line by companies, might lead to situations where repeated approval processes could become necessary. This may lead to sunk costs for the industry while rendering the approval process ineffective.
There was consensus among consulted experts that cell banking itself did not present a major IP issue for the industry as it follows established methodologies. IP primarily resides in proprietary product formulations, novel media ingredients and cell isolation and modification techniques as well as bioreactor design and process innovations further downstream for cell banking (e.g. novel materials for growth matrices). To communicate with regulators and possibly industry bodies transparently and confidentially (although the emerging CCP industry has currently no specific industry body), it was suggested that the industry may benefit from a secure, confidential platform manged by a trusted institution to facilitate knowledge sharing among the industry anonymously and with the aim to facilitate regulatory processes.
In conclusion, although regulatory standardisation is considered overall beneficial to industry growth it was emphasised that it should be driven by risk-based approaches rather than overly restrictive regulatory mandates.
8. Conclusions and discussion
Conclusions drawn in this section are based on combined findings and insights from the literature review part of this study and the expert elicitation workshop. There was generally good agreement and consistency between findings in the literature and views held by experts. Where opinions of experts are reported in the conclusions this is expressly stated. None of the conclusions represent FSA opinion.
Early process stages of cultivated meat production including cell banking practices are at present highly diverse and are evolving rapidly
Technologies for cultivated meat production are at an early stage of maturity, despite a small number of products already being approved by regulators for human and animal consumption. The laboratory methods and workflows used by different producers for cell sourcing, isolation, and banking are at present highly diverse due to the different requirements for a) cells from different source species and b) the stage of differentiation at which cells are banked (e.g. embryonic stem cells, adult stem cells, iPSCs, tissue fibroblasts). A diversity of laboratory protocols is also expected because cells of many of the species used including cattle, pig, fish, and seafood species have been studied less extensively in fundamental cell biology research in the past, as opposed to classic “model organism” cells, such as those from the mouse, fruit fly, zebrafish, frogs, and in addition, human cell lines. Hence, basic laboratory techniques for upscaled cell production methods of cells from these species are less standardised and are currently under intensive investigation by academic and commercial research to optimise methods to produce CCPs.
As many actors in the cultivated meat field are currently investing considerable efforts and resources into improving the fundamental laboratory processes to enable commercial viability of production and improvements in final product quality, it is expected that methodologies will remain diverse over the coming five to ten years due to innovations and novel process ingredients. These innovations may include the cells themselves through genetic modification approaches as well as cell growth media (aiming to avoid the use of animal sourced ingredients), growth substrates/matrices, and bioreactor design among others.
There is good awareness of general food safety aspects of cultivated meat production, but a lack of standardised production processes creates the need for more evidence-based information and guidance
Risks and hazards of CCP production processes that might impact food safety are reasonably well described in recent academic literature and are understood by producers. With information required for regulatory approval shared more widely the potential hazards considered important by regulators and what control measures are required to meet regulatory compliance in different jurisdictions are also better understood. These more general food safety risks and hazards concern mainly contamination with bio-pathogens, such as viruses, bacteria (mycoplasma and others), fungi, and prions. These pathogens can be introduced not only from the immediate processing/manufacturing environment, but also through source cells, and media ingredients as well as adhesive substrate molecules of animal origin that are introduced at the early process stages leading up to cell banking.
Testing requirements for these hazards are well understood and documented in biomedical cell banking as well as in the food sector where testing for bio-pathogens is mandatory. However, due to an absence of standardised production processes in the cultivated meat context, there is currently insufficient guidance on what specific tests need to be performed (as well as on sensitivity and specificity criteria), at which specific process steps, and how often. While producers generally concur on adhering to GMP, GLP, GCCP, and guidelines of food regulators, there are currently no benchmark “industry standards” in the emerging cultivated meat sector that would apply to specific stages of the production process including cell banking.
In addition, the science that would causally link potential effects of certain residual chemicals with impacts on human health is highly complex. The number of chemicals and molecules used in cultivated meat production is high, and chemicals such as cryoprotectants, novel media components, cell protectants to reduce damage through shear forces, and biologically active molecules such as growth factors and hormones may have effects on humans at low concentrations after long-term consumption that are currently unknown. Hence, the specific scientific evidence base on commonly used molecules and guidance on acceptable residual concentrations of such molecules needs to be established for the cultivated meat context. Experts consulted for this study agreed that more guidance on these points by regulators would be helpful for producers.
QC and food safety testing requirements for cell banking need to be better defined for the cultivated meat context and balance the setting of high standards for banking of source cells with technical and commercial feasibility for producers
As the production methodologies for cultivated meat originate in biomedical laboratories, the regulatory demands for biomedical applications are well understood and are particularly high when cells are used for therapeutic purposes in humans. For the cultivated meat context, it is currently not clear which parts of the production process would benefit from stricter regulatory frameworks, and which aspects of production processes could be carried out under less stringent regulation, given a food context. For example, for possible future GMO, authenticity, origin tracing or other regulatory labelling requirements, initial production stages might have to be more rigorously documented in a more standardised manner and specific QC processes carried out to ensure the quality and authenticity of used cells. This might be of particular relevance for early process stages such as cell banking, as it is the foundational step that defines the quality of the starting material (cells) of the production process.
However, experts consulted for this study agreed that a balance needs to be achieved between required depth of testing and commercial feasibility of testing procedures. Hence, a widely held opinion among experts is that the most in-depth testing and cell characterisation should be carried out at, or in parallel with cell banking, i.e. when the source cell lines that are used for production of CCPs are established and banked. A second set of rigorous tests assessing food safety risks and cell authenticity/genetic stability should be carried out at the finished product stage. Currently the level of testing in terms of methodological depth and statistical sampling criteria is not well defined for cultivated meat production processes; experts consulted for this study believed that guidance on these aspects from regulators would be helpful for producers.
Experts consulted for this study also agreed that for regulators to be able to do this, further research is required to determine the extent of acceptable residual molecule monitoring necessary for downstream processing steps, particularly when final products are consumed in raw or semi-raw forms such as in sushi or sashimi, or rare-/medium-rare meat preparations.
Requirements for cell origin and authenticity tracing and necessary technology solutions are currently not well defined for the cultivated meat context
The importance of (cell) traceability and documentation systems is well understood in the biomedical field as well as in the food sector where origin tracing are legal requirements. However, there are currently no agreed upon standards and methodologies for the tracing of cells from origin and throughout the cultivated meat production process. Experts consulted for this study agreed on the need to establish robust tracing and tracking methodologies for cells (and source animals if derived from biopsy or tissue sample) used in cultivated meat production processes. Some of the solutions discussed by experts were:
-
Use of currently available veterinary documentation information and systems for documenting origin of cells that originate from animals already in the food system
-
Application of blockchain technologies or other safe digital methods to trace cells from cells banks and to the source of cell origin (e.g. individual animal that was the source of biopsy)
-
Use of robust and simple molecular markers for banked cells and QC of final product (e.g. STR testing)
Experts also agreed that the emerging industry still needs to establish efficient and cost-effective tracing/testing methods for large-scale CCP production processes. These could then be used more widely to harmonise cell origin traceability practices in the cultivated meat sector in the future. When cells are sourced (via biopsy/tissue sample) from animals that are already part of the food system, existing veterinary and other origin tracing systems should be utilised, and relevant data used in any additional downstream cell tracing systems. When cells are purchased from commercial cell providers, it is currently not clear what documentation they would need to transfer to the cultivated meat producer so that the producer can operate within legal tracing requirements.
Cell origin tracing may also be more challenging for some product types such as in the sea food sector, as mostly cells are sourced from wild-caught animals. Therefore, adaptation of existing food industry documentation systems with consideration for novel regulatory demands for use of these cells in the cultivated meat sector was considered advisable. Experts consulted for this study also agreed that standardising requirements for the documentation of cell origin and traceability will enhance transparency and facilitate regulatory approval process as well.
Explicit information on cell banking procedures, and cell banking media ingredients is currently limited, and no standardised definitions of master cell bank/working cell bank are established for cultivated meat production processes
Explicit information on cell banking practices within the cultivated meat context is very limited in the academic literature and can only be inferred indirectly from occasional academic publications mostly with a focus on recent innovations. Experts consulted for this study believed that currently used cell banking practices are considered “standard” methodology by producers, unlikely to undergo significant innovation or yielding any protectable IP. However, the specifics and details of cell banking protocols can vary considerably depending on the cell type (different types of stem cells) or the species of origin.
From academic and industry publications on cultivated meat it is often unclear at what stage a cell bank is considered the master cell bank, or whether several additional cell banking steps are carried out for different purposes further downstream, and what their specifics are. Hence, clearer definitions for the types of cell banking carried out specifically in the cultivated meat context need to be developed to support also regulatory decision making on QC requirements of cell banking. Although definitions for Master Cell Bank (MCB) and Working Cell Bank (WCB) are well established in the biomedical field, there is a need to consider definitions for the context of cultivated meat production processes. Experts consulted for this study agreed that such clearer definitions would also support regulatory guidance on cell banking and approval processes.
The role of genetic stability/genetic drift of cells used in cultivated meat production as a source of risk for humans is scientifically not understood (although considered likely to be low by experts) - and monitoring and testing requirements for genetic drift are currently undefined
Genetic change/drift over time is a normal phenomenon in all cells of an organism as well as in cells maintained in cell culture in a laboratory. Currently, there is no systematic evidence base that documents possible rates of genetic change specifically of cell types/species used in cultivated meat production. In addition, results from other research contexts investigating genetic drift might not be easily transferable, as the scale and specifics of cell culture practices used in cultivated meat production might directly impact rates of genetic change of the specific cell types used.
Academic literature and experts consulted for this study indicate that the specific cell type, its differentiation state (e.g. embryonic stem cell, adult stem cell, iPSC, resident tissue cell), and the specific growth conditions (e.g. media, growth substrates, incubators used) all are likely to impact rates of genetic drift. Moreover, it needs to be considered that most cultivated meat products consist of several cell types, grown either separately or together making definitions of an overall “acceptable” rate of genetic drift even more complex. Currently, there is no baseline or reference data available on “normal” rates of genetic change in cell types used under the respective specific cell culture conditions.
In addition, there is no scientific evidence base available that would link specific genetic mutations with potential risks for humans when ingesting cultivated meat products. Although potential concerns around genetic drift changing the allergenicity of abundant proteins in muscle cells (e.g. tropomyosin and parvalbumin - known allergens in fish and seafood for some people) were discussed by experts, they also confirmed that scientific evidence on the likelihood of such events and how they might affect humans with allergies is currently lacking. However, methods recently developed to predict allergenicity of other novel food products using in silico approaches might be applicable to the cultivated meat sector (Liguori et al., 2022; López-Pedrouso et al., 2023; Westerhout et al., 2019).
Experts consulted for this study suggested that a pragmatic approach for testing genetic drift/cell line stability was advised, prioritising production consistency rather than extensive genetic characterisation. Phenotypic assessments were deemed more appropriate for day-to-day monitoring, as they are cost effective and proven sufficient for product consistency. By establishing definitions of acceptable phenotypic variation in cells of the final product, and focusing on overall phenotype level testing rather than exhaustive genomic analysis at every stage, robust testing standards that are feasible for producers might be developed.
Despite diversity and a currently dynamic evolution of cell culture and banking techniques for cultivated meat production, regulatory guidance on specific aspects of current practices might support growth of the emerging CCP sector
Most experts consulted for this study agreed that currently there are no “standard” practices established for early process stages, including cell banking. While they also suggested that regulatory guidance on some specific aspects of the cultivated meat production processes would be beneficial in shaping the emerging industry, it was emphasised that such guidance should be founded on risk-based approaches rather than overly restrictive regulatory mandates. Rigid characterisations/definitions of processes and workflows were also considered impractical because currently a diverse and evolving set of practices is used to produce similar finished cultivated meat products. Areas where more regulatory input was perceived as helpful for the industry included the development of guidance on permissible residual level of media and process components, particularly due to potential presence of bioactive molecules such as hormones and growth factors in final products. Specifically for the cell banking stage, experts thought that bio-pathogen testing would be most essential, but currently guidance on statistical sampling of cell banks, types of tests, frequency, and test specificity/sensitivity, is limited. General guidelines on definitions of what constitutes a master cell bank/working cell bank in a cultivated meat production context were suggested to be helpful also for regulatory approval purposes.
Minimum required tests for cell line characterisation and level of depth of characterisation might need consideration regarding compliance with future labelling requirements and emerging QC standards. As currently no standards of cell origin tracing, and tracing through the production process are established, it was suggested that framework guidance on required tracing methodologies and systems (e.g. which tests, documentation systems and data are used) would help standardise practices, as tracing of food and ingredients is a legal requirement in the food sector.
9. Recommendations to the FSA
The following recommendations to the FSA are proposed by the authors of this report after analysis of the academic and grey literature and the outputs of the expert elicitation workshop. These recommendations are given with consideration of the regulatory remit of the FSA, however, do not express FSA opinion or intent of future policy.
Published academic and industry literature on key information of cultivated meat products with regards to ingredients is still very limited (a point that a previous FSA report on the identification of hazards in meat products manufactured from animal cells has already made in 2023). This might not only be because of confidentiality issues on behalf of producers, but also because the field is currently rapidly evolving. Moreover, the science base underlying cultivated meat production processes is complex. Hence the following recommendations are given with a view to addressing several knowledge and evidence gaps identified in this study and to help with identifying necessary evidence for longer-term regulatory decision making.
For FSA to establish the relevant evidence base for assessing products submitted for future approval, it might need to interact more proactively with major R&D players and producers in the cultivated meat space. As the field of active players is currently still small, such interactions would be manageable in a timely fashion to help shape the emerging sector. Such interactions could be facilitated via themed expert workshops, or web platforms that are dedicated to specific issues that need to be better understood in the cultivated meat production context (e.g. toxicology, allergenicity, bio-pathogen testing among others).
As cell banking is a crucial stage for QC and hazard testing of used cells in the production process, FSA might wish to investigate current cell banking practices in the biomedical sector in more depth, with a view to establishing realistic and safe regulatory requirements that are appropriate for the food context.
Despite that diverse approaches exist to cell line establishment and cell banking enough producers are now seriously pursuing large scale production, and hence, more detailed information might now be available.
This might be the right time for the FSA to engage with producers to establish a database of most commonly used chemicals and molecules used in CCP production. FSA might set up formats of engagement with industry that protects commercial confidentiality and proprietary information but encourages sharing of information to help streamline industry practices.
As now a larger number of cultivated meat producers are active and R&D literature is becoming available:
FSA might wish to collect the relevant evidence base for establishing risk profiles and minimal residue concentration requirements for cultivated meat products. This might help also with building trust in consumers and facilitate product approval processes.
Cell culture/banking and production practices are expected to evolve dynamically over the coming years, particularly in the field of novel cell culture media ingredients and growth substrates (of plant, animal and synthetic origins). It is important that innovators in the cultivated meat sector are made aware early in the innovation process that food ingredients with no history of use prior to 15 May 1997 must be assessed for approval by FSA under Assimilated Regulation 2015/2283 on novel foods. The exceptions to this legislation include food enzymes, food additives and flavourings, and extraction solvents which are subject to their own legislation.
Hence, it might be beneficial for the emerging industry that FSA communicates the legislative position on such novel ingredients more explicitly for processes involving cell culture/banking for CCP production, as practitioners in this technology area may not be aware of the relevant extant legislative processes relating to their work.
Any concerns regarding the possibility that genetic stability/drift of cells might pose risks to humans (e.g. allergenicity) can currently not be backed up with scientific evidence (although consulted experts considered the risks to human health as unlikely). This includes concerns that some cells may develop cancerous characteristics or increased allergenicity of proteins. However, whether the consumption of such cells in cultivated meat products might have any impact on human health is currently not studied. Should there be an intention to address such concerns via regulatory guidelines in the future, this would also raise questions around specific detection methods for substantially different cells and the capabilities needed to do so by labs serving enforcement authorities.
For FSA to be able to provide guidance on acceptable rates of genetic or phenotypic change of cells and potential impact of such change on human health, it is recommended to carry out a separate study on this topic. This should help FSA to assess how much effort, and resources might be required for regulatory decision-making in this matter.
Currently, there are no standard practices for tracing cell origin and tracing of various cell types throughout the entire production process. Different producers may currently address this in different ways and no agreed upon approaches are reported form within the emerging industry. However, early engagement with commercial and academic R&D players might help find appropriate solutions that might also streamline approval processes.
For FSA to be able to develop appropriate traceability (authenticity) requirements of cells for cultivated meat products, it is advisable to investigate to what extent cell tracing in the biomedical sector and existing tracing technologies already existing in the food sector might be used to set feasible standards for CCPs. Such efforts would require interdisciplinary evidence gathering also with regards to risk assessments of potential hazards that may arise from a lack of cell tracing and documentation.
Clarity on this matter should also help to define responsibilities technical solutions within FSA regarding whether traceability is more important for labelling and authenticity requirements, or whether food safety concerns are a priority.


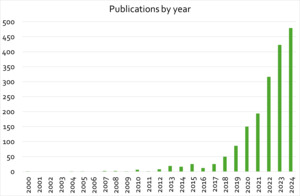


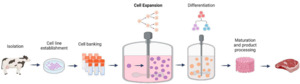
_and_induced_pluripotent_stem_cells_(ipscs)_are_derived_from_adult_.png)
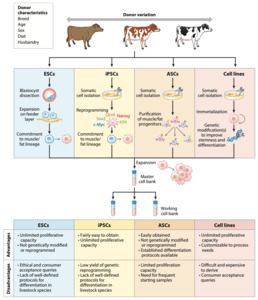


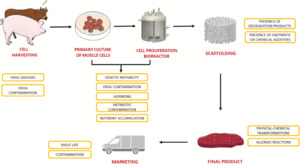
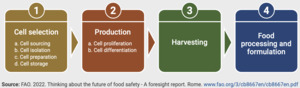
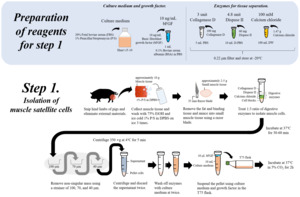
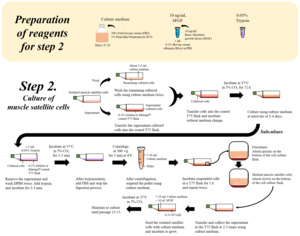
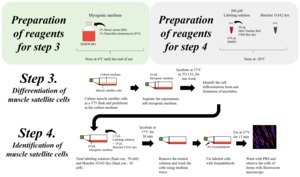









.png)
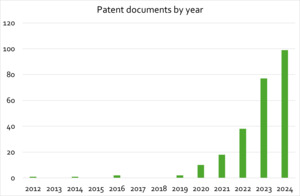

._nl_th.png)
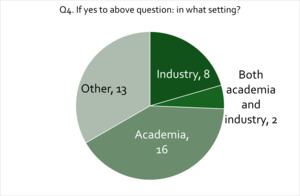
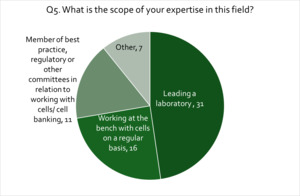
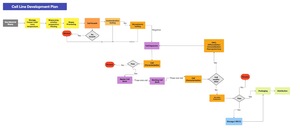


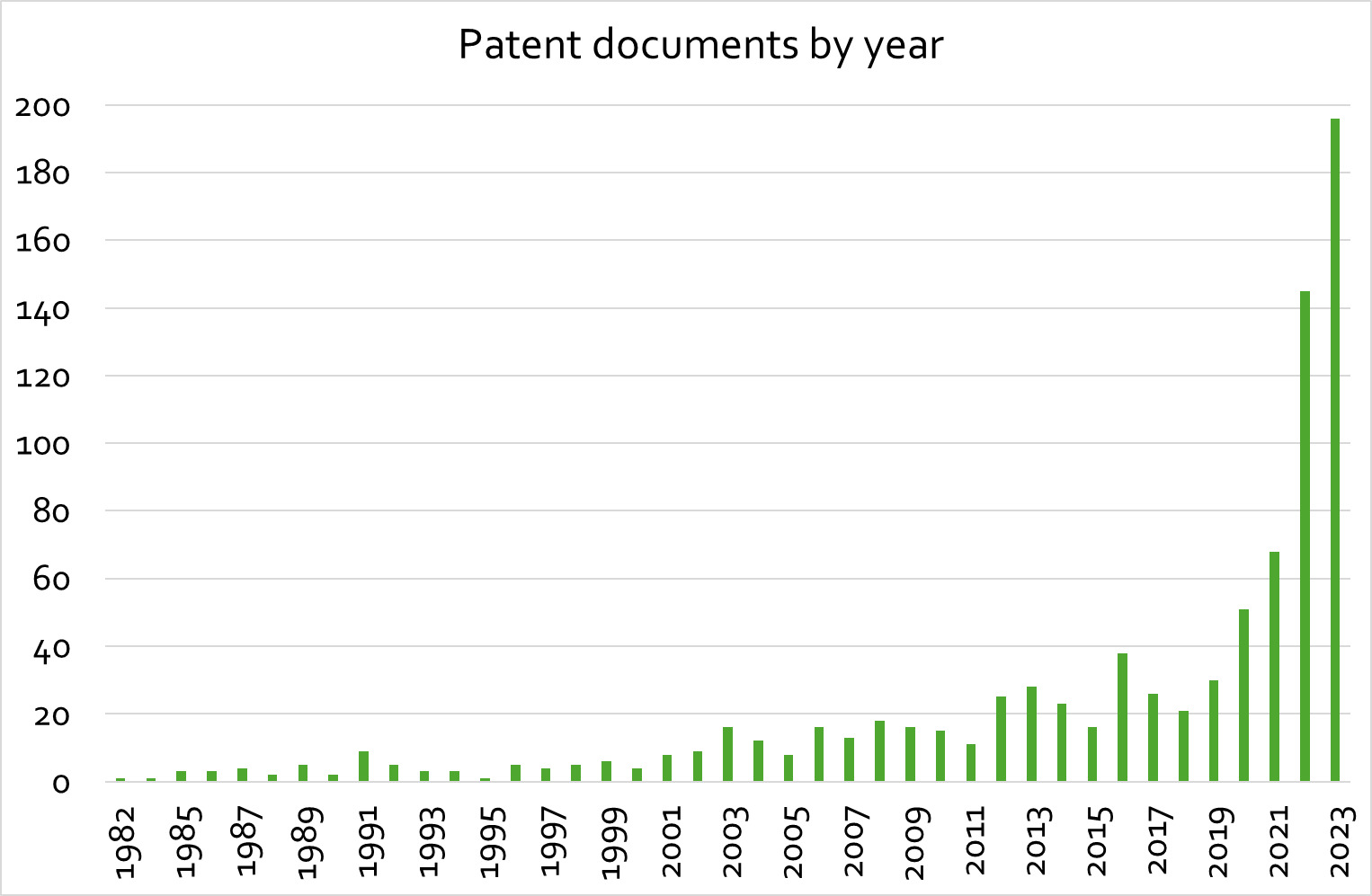
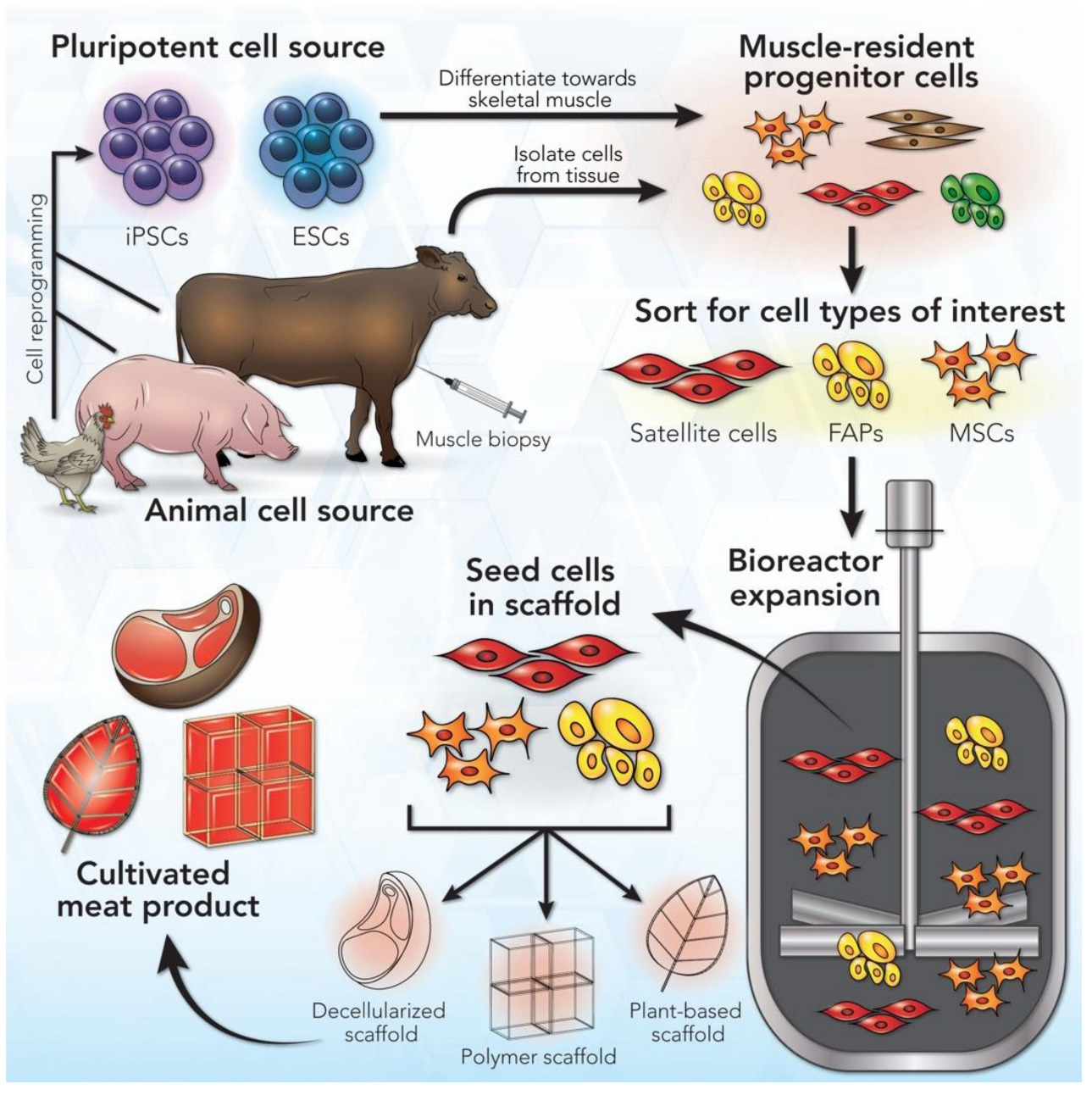

_and_induced_pluripotent_stem_cells_(ipscs)_are_derived_from_adult_.png)

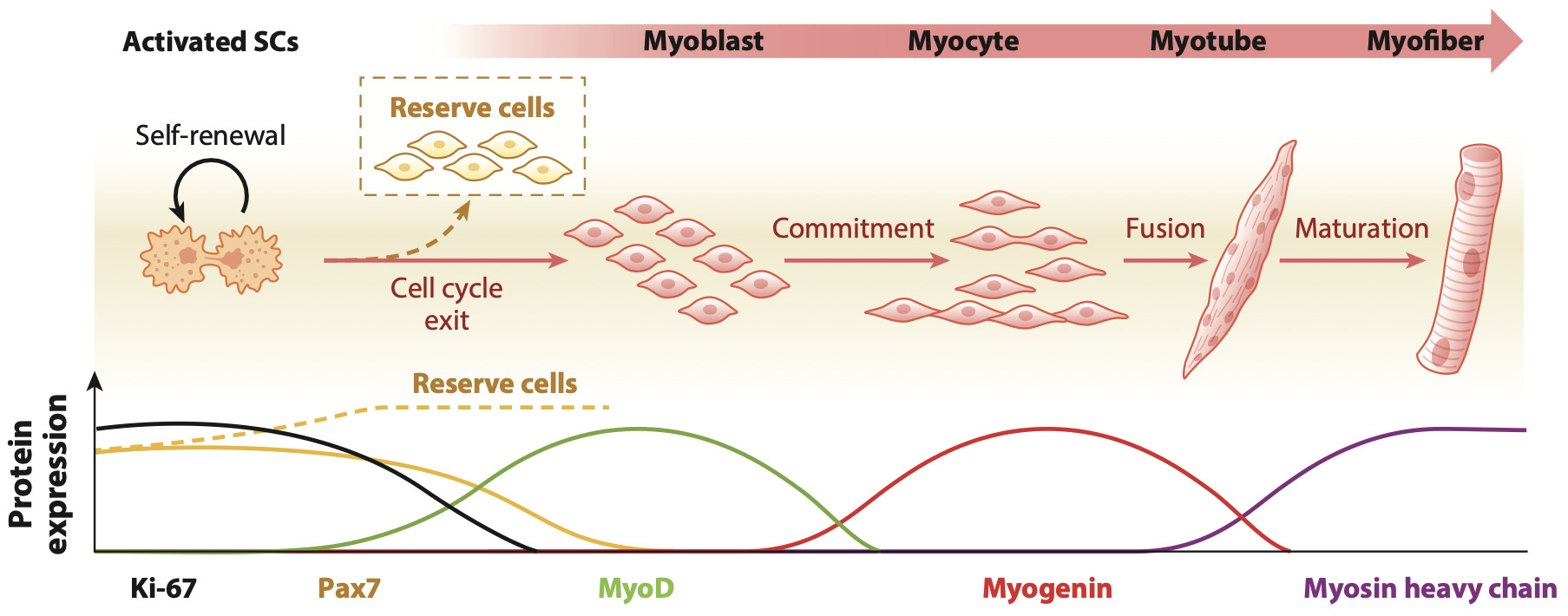

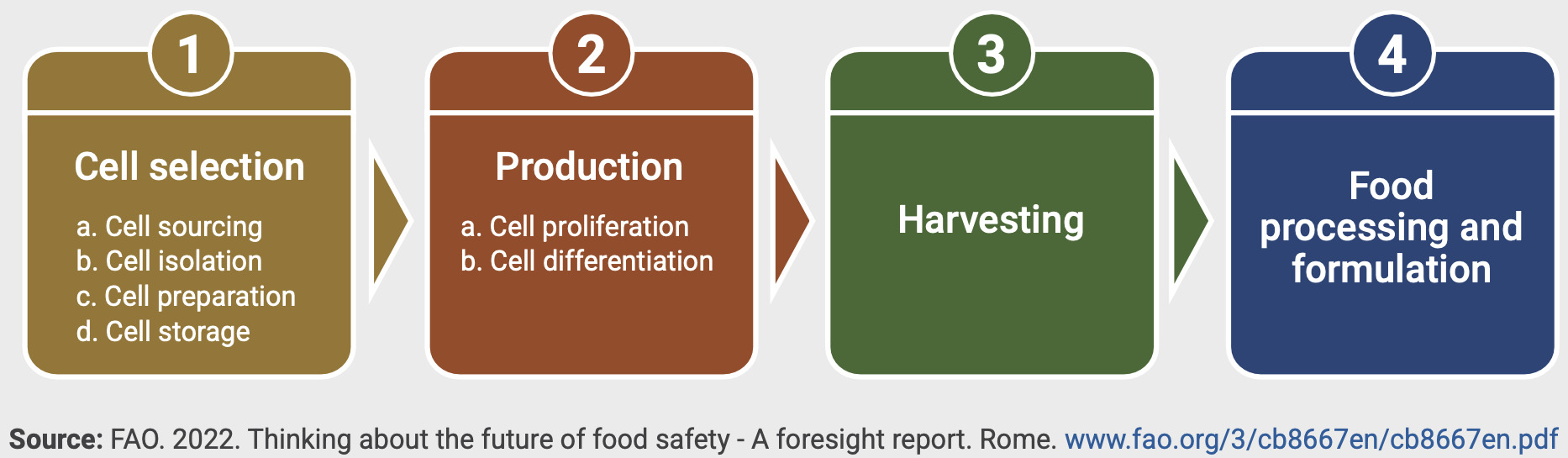
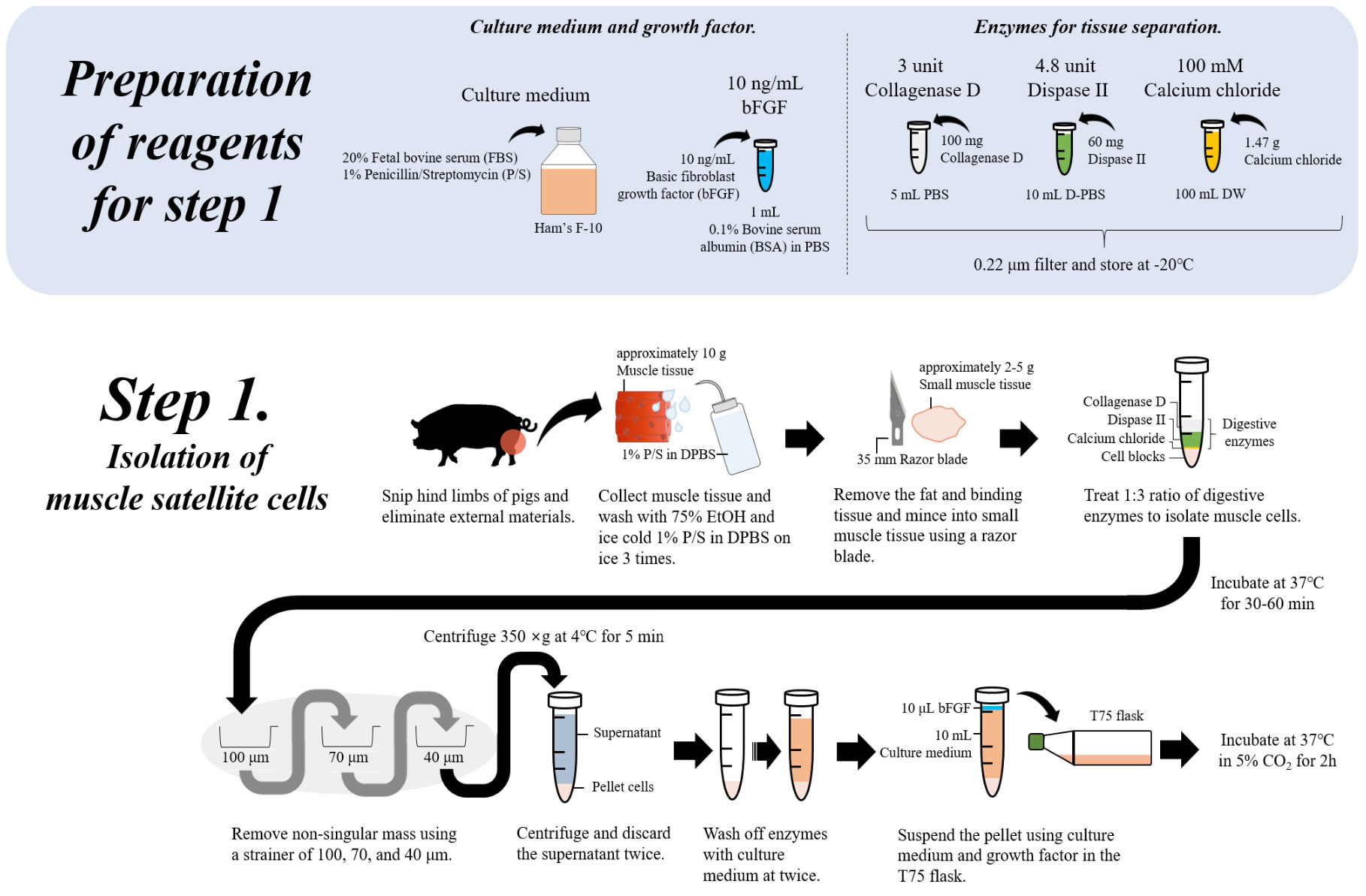
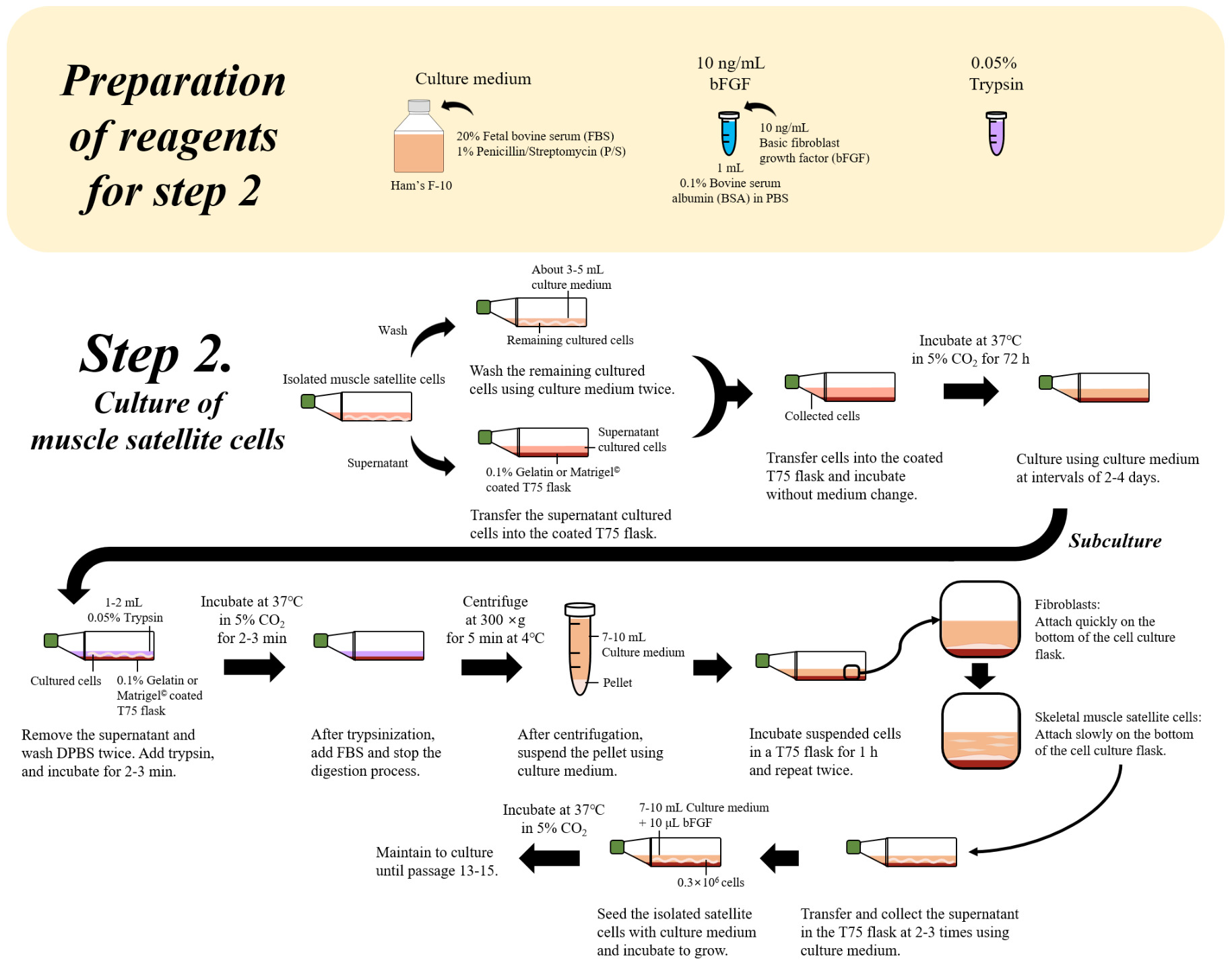


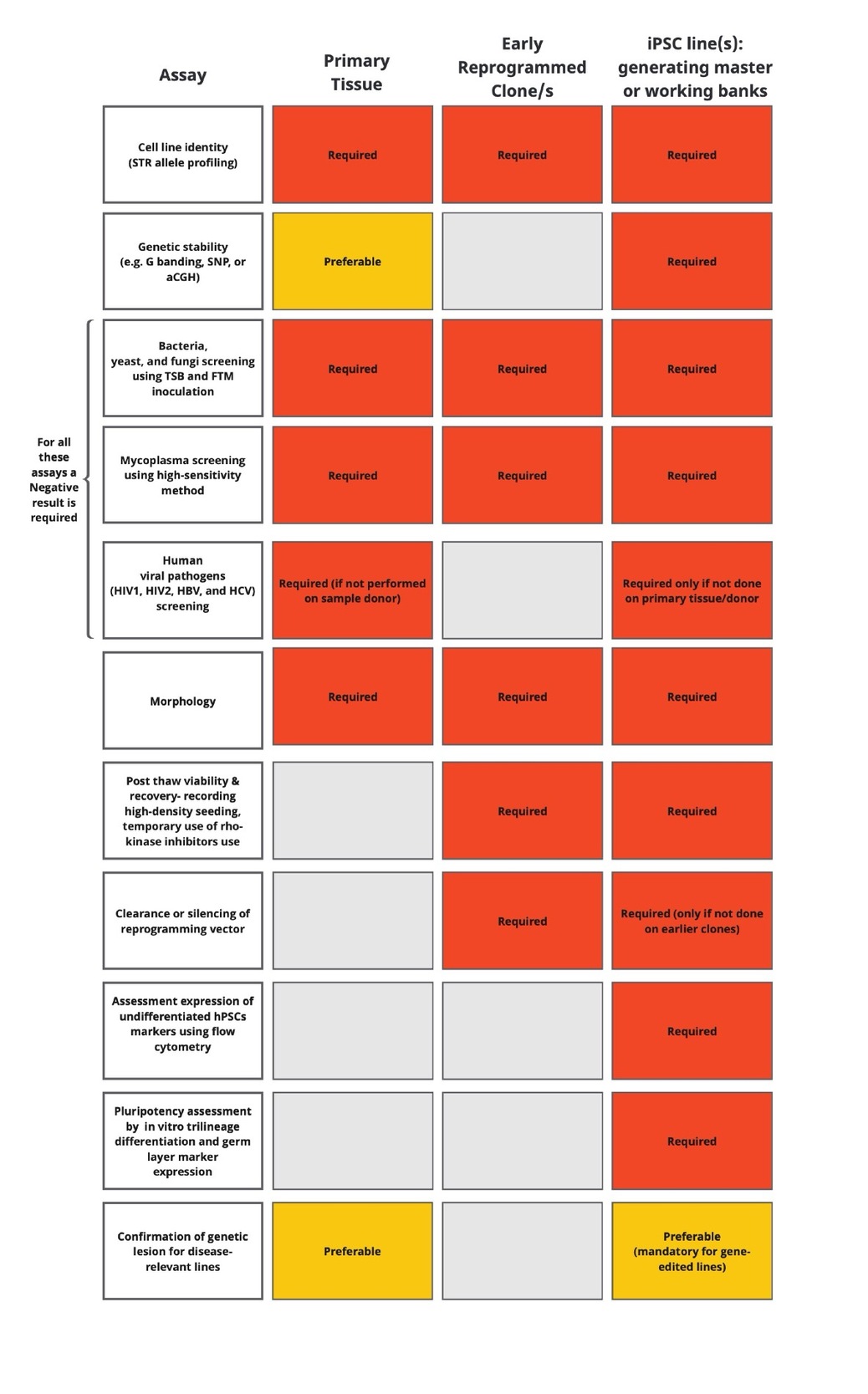

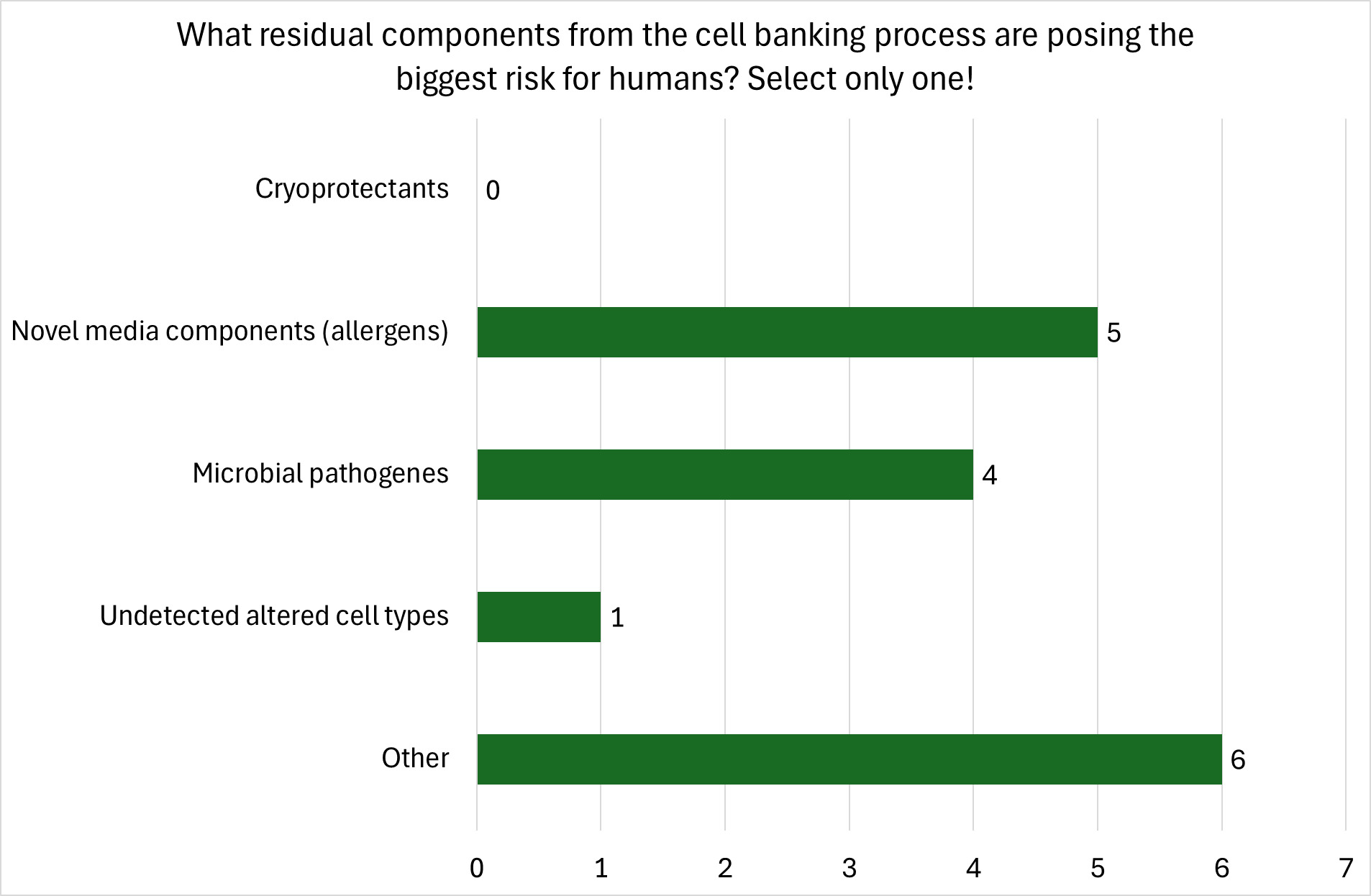
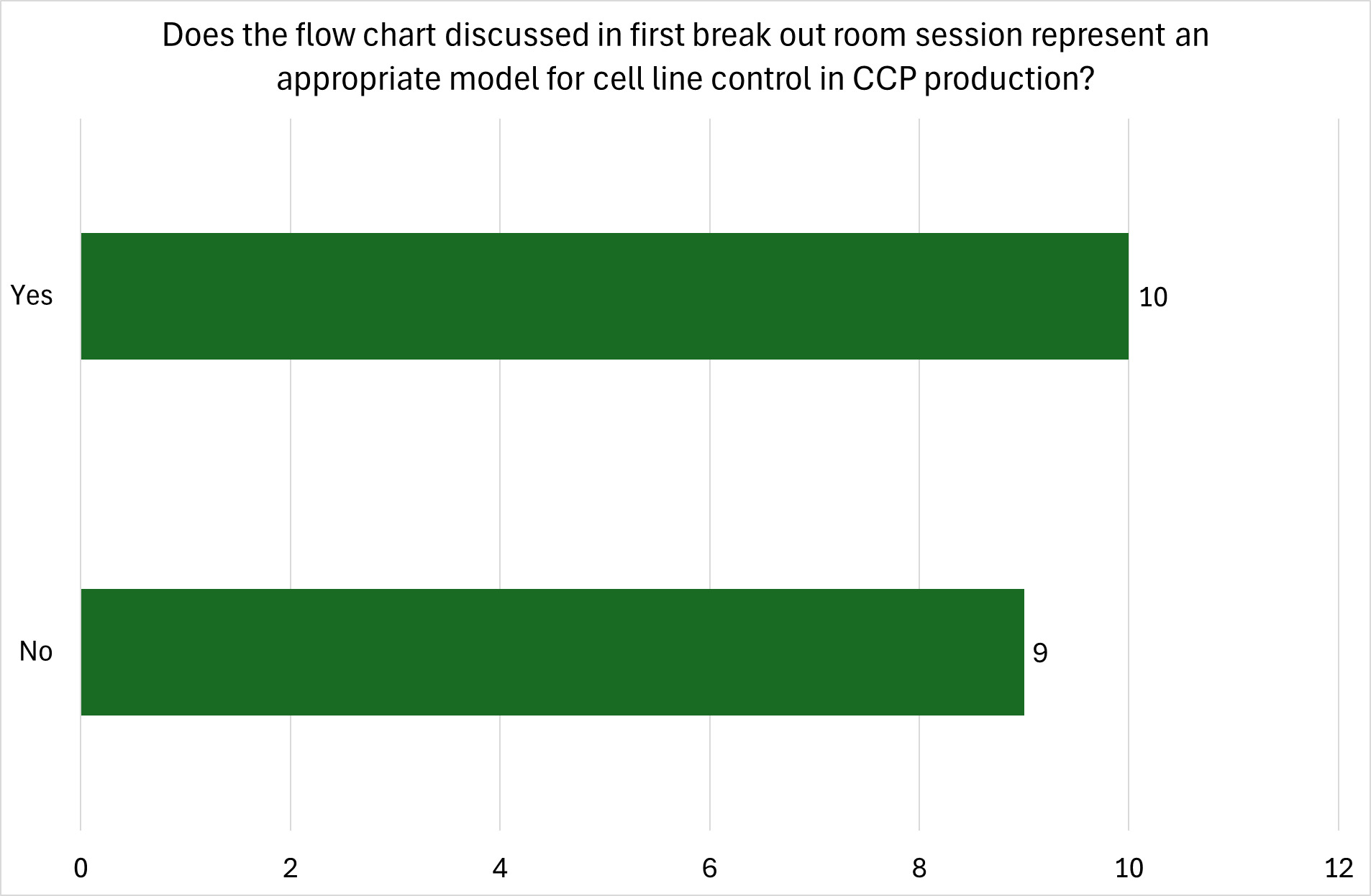

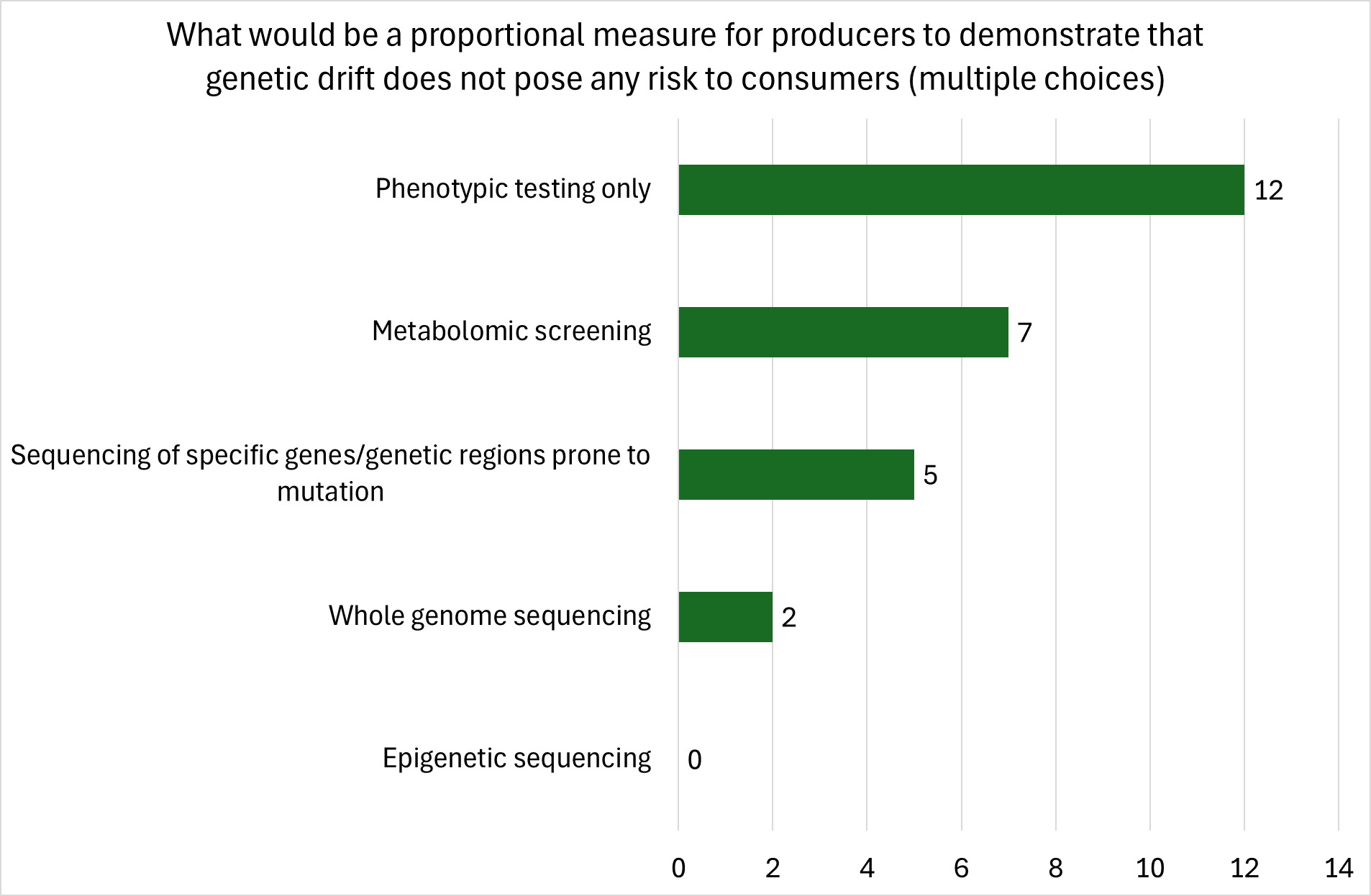


.png)


._nl_th.png)