Abbreviations
ADI Acceptable Daily Intake
ADME Absorption, Distribution, Metabolism and Excretion
AHR Adjusted Hazard Ratio
AMSTAR A Measurement Tool to Assess Systematic Reviews
BMI Body Mass Index
CI Confidence Interval
COC Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment
COT Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment
Cys-SNO S-nitrosocysteine
EFSA European Food Safety Authority
FFQ Food Frequency Questionnaire
GB Great Britain
GI Gastrointestinal
GRADE Grading of Recommendations, Assessment, Development and Evaluations
GSFA General Standard for Food Additives (Codex)
HR Hazard Ratio
IBD Inflammatory Bowel Disease
JECFA Joint FAO/WHO Expert Committee on Food Additives
MAM Multiplaque Artificial Mouth
Mb Myoglobin
MCFA Medium-Chain Fatty Acids
NAC-SNO S-nitroso-N-acetylcysteine
NACET-SNO S-nitroso-N-acetylcysteine Ethyl Ester
NDMA N-nitrosodimethylamine
NIH-AARP National Institutes of Health-American Association of Retired Persons
NMOR N-nitrosomorpholine
NMPhA N-nitroso-methyl Phenylamine
NN Nitrate or Nitrite Additives
NO Nitric Oxide
NO-AcTrp N-acetyl-N-nitrosotryptophan
NOCs N-Nitroso Compounds
NPIP N-nitrosopiperidine
NPYR N-nitrosopyrrolidine
OR Odds Ratio
PWMP Plasma-treated Winter Mushroom Powder
RASFF Rapid Alert System for Food and Feed
RQ Research Question
SCF Scientific Committee on Food
SCFA Short-Chain Fatty Acids
SETE Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup of the Committee on Toxicity and the Committee on Carcinogenicity
SHIME Simulator of the Human Intestinal Microbial Ecosystem
1. Introduction
1.1. Project background and terms of reference
RSM UK Consulting LLP (RSM) in conjunction with Professor Gunter Kuhnle (University of Reading), Dr Duane Mellor (University Hospitals of Leicester NHS Trust, also affiliated with Aston University and University of Canberra) and University of Birmingham Library Services, were commissioned by the Food Standards Agency (FSA), to carry out a literature review on the safety of nitrates and nitrites as food additives.
The aim of the study was to review the current scientific literature on the safety of four approved nitrates and nitrites as food additives. This includes the absorption, digestion, metabolism and excretion (ADME) processes, toxicological profiles, and prevalence in food for human consumption. The findings would update the FSA’s knowledge, aiding in risk assessments, risk management, and regulatory decisions, to ensure food safety for UK consumers.
This was a short-term project (~3 months). Therefore, the focus of the review was from 2016 to present intended to capture the literature published since the 2017 re-evaluation of nitrates and nitrites as food additives by the European Food Safety Authority (EFSA) (Mortensen et al., 2017b, 2017a).
1.2. Summary of previous evaluations on the safety of nitrates and nitrites by international regulatory bodies
Nitrates (sodium nitrate or E 251 and potassium nitrate or E 252) and nitrites (potassium nitrite or E 249 and sodium nitrite or E 250) are salts commonly used as food additives for their antimicrobial effects, as well as their ability to maintain properties such as colour, texture and flavour. Several international regulatory bodies have previously conducted evaluations on the safety of nitrates and nitrites. This section provides a summary of key findings from these evaluations.
International Agency for Research on Cancer (IARC)
In 2010, the IARC evaluated the carcinogenicity of ingested nitrate and nitrite and their impact on cancer risk (IARC, 2010). While they found inadequate evidence for nitrate and limited evidence for nitrite with regard to their carcinogenicity from food, they concluded that ingested nitrate or nitrite are probably carcinogenic to humans (Group 2A).
The rationale for this decision was based on the conversion of nitrate and nitrite to carcinogenic N-nitroso compounds (NOCs) in the body. Humans have an internal nitrogen cycle where nitrate and nitrite are interconverted, and under acidic stomach conditions, nitrite can form nitrosating agents. These agents react with certain compounds to produce NOCs, some of which are known carcinogens.
EFSA
The safety of nitrates and nitrites themselves as food additives was last comprehensively evaluated by the EFSA in 2017 (Mortensen et al., 2017b, 2017a). EFSA concluded that nitrates and nitrites as food additives were safe at the permitted levels and conditions, and agreed on an acceptable daily intake (ADI) for nitrates of 3.7 milligrams per kilogram of body weight per day (mg/kg bw/day) and of 0.07 mg/kg bw/day for nitrites.
In 2023, EFSA evaluated the safety of nitrosamines, which can form from nitrates and nitrites (EFSA CONTAM Panel, 2023). They concluded that current exposure levels to nitrosamines are a health concern, leading to stricter limits being set on the use of nitrates and nitrites as food additives by the European Commission. EFSA maintained the ADI for nitrates at 3.7 mg/kg bw/day and re-established the safe level for nitrites at 0.07 mg/kg bw/day. Following on from this evaluation, the EU announced a decision to change the maximum permitted levels of nitrites and nitrates used as food additives to levels lower than those allowed in Great Britain (GB). This prompted a review of the current understanding of the safety of these additives in food sources in the context of GB legislation.
Food Standards Australia New Zealand (FSANZ)
FSANZ (2011) conducted an analytical survey estimating dietary exposure to nitrates and nitrites. The study focussed on food and beverages in Australia and was not limited to food additives. In May 2010, food regulatory agencies in the Australian Capital Territory, Western Australia, and Queensland collected 52 food items to measure their nitrate and nitrite content. To estimate dietary exposure, dietary modelling methods were employed, which involved combining national nutrition survey data with the nitrate and nitrite concentration data from the collected food items.
The evaluation found that the highest concentrations of nitrates were present in fruits and vegetables, while processed meats had the highest concentrations of nitrites. In terms of dietary nitrate and nitrite intake, fruits and vegetables were major contributors to the overall intake (11-30% and 42-78%, respectively). Infants aged nine months had the lowest estimated dietary exposure to nitrate and nitrite, whereas those aged 17 years and above had the highest. Despite the varying exposure across age groups, the research findings indicated that dietary intake of nitrates and nitrites did not pose a significant health risk to the Australian population. FSANZ recommended increased consumption of fruits and vegetables, concluding that the health benefits, including cancer and cardiovascular disease prevention, far outweigh the health risks linked to dietary nitrate and nitrite intake.
Health Canada
Health Canada (2013) conducted a comprehensive review to identify health risks associated with nitrate and nitrite in drinking water, food and the environment. This research found that nitrate and nitrite are commonly present in vegetables and cured meats, such as sausages, luncheon meats, and cold cuts. A study found that nitrate present in food may lead to reduced endogenous formation of carcinogenic NOCs in the body compared to nitrate in drinking water. This is because dietary antioxidants, such as vitamin C found in certain fruits and vegetables, have protective factors against cancer. Endogenous formation occurs within the body when nitrate is reduced to nitrite by oral bacteria, which can then form NOCs in acidic gastric conditions.
Across studies, health risks related to nitrate and nitrite exposure included methaemoglobinaemia, type 1 diabetes and brain tumours. Infants consuming soy-based formula or formula prepared with water containing high levels of nitrate were also found to be at increased risk to excessive nitrate exposure. Research indicated that variable stomach pH (2-5) in infants may also increase the growth of nitrate-reducing bacteria, which in turn increases the risk of forming methaemoglobin.
Health Canada identified associations between nitrite intake and gastric cancer risk. Additionally, there was evidence suggesting that nitrate exposure may affect thyroid function. This is particularly concerning for pregnant women, as it can impact foetal development. Health Canada recommended regular monitoring of nitrate and nitrite levels in drinking water and food to safeguard public health. As part of their ongoing guideline review process, Health Canada monitor new research into health effects associated with nitrate and nitrites.
Joint FAO/WHO Expert Committee on Food Additives (JECFA)
JECFA commissioned two evaluations exploring the safety of nitrates and nitrites as food additives and their role in the formation of NOCs. These evaluations reviewed data from studies on the adverse effects of nitrate and nitrite consumption and modes of action, both in humans and animals. The first evaluation, focused on nitrates, concluded that the toxic effects of nitrates were largely due to their conversion to nitrites in the body (Speijers & van den Brandt, 2003a). Studies have estimated that in humans nitrate is secreted in saliva in a dose-dependent manner, with about 25% of ingested nitrate being secreted in saliva. There was mixed evidence regarding the link between nitrate in drinking water and risks for prostate cancer and brain tumours.
The second evaluation, focused on nitrites, found that nitrite can induce methaemoglobinaemia (Speijers & van den Brandt, 2003b). Contamination of food was a key factor in this context, including excessive levels of nitrite in food items such as dim sum and Chinese sausages. Some studies found increased risks for oesophageal and gastric cancer, related to nitrite intake while others revealed no association. Future research into the adverse effects occurring from short-term exposure to nitrite was recommended, as the studies included in this evaluation were primarily related to its long-term toxicity, with studies ranging from 12 months to 2 years.
1.3. Comparing GB legislation with other countries
Different jurisdictions have unique regulatory bodies and frameworks that assess and manage food safety and, as a result, permitted levels for the use of nitrates and nitrites as food additives vary across countries. Furthermore, while the WHO has established guideline values for nitrates and nitrites in drinking water (World Health Organization, 2022), it has not set specific guidelines values for their presence in food. However, the General Standard for Food Additives (GSFA) has set specific limits on the use of nitrates in ripened cheese (35 mg/kg) and the use of nitrites in processed meats (80 mg/kg) (FAO and WHO, 2025).
While permitted levels may vary between jurisdictions, they have largely remained consistent over time for countries such as Australia and New Zealand, Canada, Japan and the USA. However, as a result of the recently announced changes to permitted levels in the EU (“Commission Regulation (EU) 2023/2108,” 2023), from October 2025 GB will have less stringent limits on the use of nitrates and nitrates compared to the EU. This difference in regulation could impact food producers in GB who export products to the EU that contain nitrates and nitrites as food additives.
A direct comparison between permitted levels in GB and other countries is more challenging given that different countries regulate different food groups. However, one food group regulated by all countries in scope was cured meats.
Table 1 shows the regulation of nitrates and nitrites in cured meats. Where a range is shown, this is due to their different permitted levels for different cured meat types.
As expected, the new levels within EU regulation for nitrates and nitrites are marginally lower than in GB for some cured meat. The levels within Canadian nitrate regulation and the regulation of nitrites in Australia and New Zealand are also lower than the regulation in GB. In contrast, the levels within US regulation of sodium nitrite and nitrate are higher than those in GB. Furthermore, the levels within GB regulation are lower for nitrites than Canada, and lower for nitrates than Australia and New Zealand. It is not possible to undertake direct comparisons between GB and Japanese regulations as the levels specified in Japanese regulation are residue limits, whereas in GB they are limits to the level of nitrates and nitrites added during manufacturing.
1.4. Study aims
The primary purpose of this study was to conduct a literature review of the most up-to-date scientific literature of the four currently authorised nitrates and nitrites permitted as food additives and their safety to the consumer. This will provide an updated evidence base on the ADME processes in the human body, the toxicological profile of these compounds and their metabolites and the level of their occurrence in all applicable food categories for human consumption. This study had the following aims.
-
Develop a search protocol ensuring that all relevant studies on the safety of nitrates and nitrites as food additives are captured;
-
Conduct a literature search, screening and quality appraisal of identified papers and establish the evidence base (and thus evidence gaps) relevant to the priority research questions;
-
Extract data and synthesise findings summarising the themes from the evidence base, assessing the current understanding against each research question and the requirements for further research; and
-
Report findings to the FSA and publish the report of the literature review.
The outcomes of this work will be used to update the FSA’s knowledge of these food additives and to inform future risk assessments, risk management and wider regulatory policy decisions and guidance. Ultimately, the aim is for the information within this report to contribute to the FSA’s own mission to ensure food is safe for UK consumers.
1.5. Research questions
The literature review sought to address the following research questions:
-
What are the ADME processes relevant to human exposure of nitrates and nitrites as food additives and their metabolites? What is the effect of an individual’s microbiome on nitrates and nitrites ADME?
-
What is the toxicological profile of nitrates and nitrites as food additives for all endpoints and mechanisms of action, especially the potential link between nitrates and nitrites intake from food sources and the development of cancer?
-
What are the effects of different types of cooking and processing on the conversion from nitrates and nitrites to NOCs in food, including N-nitrosamines?
-
What is the occurrence of nitrates and nitrites as food additives in all currently authorised and non-authorised food categories?
-
What are the alternative food additives/ingredients possessing nitrates and nitrites multifunctionality and what is their risk profile?
2. Methodology
2.1. Project initiation
A project initiation meeting was held with the FSA project team on 18th December 2024. At this meeting, the following parts of the research project were agreed:
-
research approach
-
project plan
-
project management arrangements
-
reporting plan
-
risk management procedures
-
mapping relevant stakeholders to invite them to join the expert panel
An expert panel was created to support with different elements of the research, including refining the draft search protocol, signposting the research team to literature (academic and grey literature) which may be relevant to this study, discussing emerging findings from the review, commenting on completeness and gaps and providing input to the development of conclusions for the report. This included representation from members of the FSA policy team as well as experts from the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT).
2.2. Scope of the review
The scope of the review was clearly defined to be proportionate to the resource and time limitations.
-
For this project, nitrates and nitrites referred to the following four compounds: potassium nitrite (E 249), sodium nitrite (E 250), sodium nitrate (E 251) and potassium nitrate (E 252).
-
The term “food additive” referred to cases where the compounds are added into food sources. This could include naturally occurring nitrates and nitrites used directly or indirectly (for example, through concentration) by the food industry to create, enhance or process food. Studies were excluded if they focussed on nitrites and nitrates naturally occurring in food, or in other words, cases where the compounds have not been externally added.
-
Studies examining additives in drinking water and other drinks/beverages were excluded, as the review focussed on additives in food.
-
Findings from animal studies were also excluded. The focus of the review was on human health and due to time and resource constraints and the reasonable availability of human data through epidemiological and in vitro models, animal studies, although identified in the literature search, were not included in the review.
-
When considering alternatives, studies were included if they explored properties, risks or benefits of alternative additives or ingredients to food substances. Studies that investigated other alternatives that were not food-based, such as active or intelligent packaging solutions, were excluded.
-
For the purpose of this project, ‘microbiome’ included both the gut and mouth microbiomes.
Each of these points above supported the screening process (described in section 2.3 below) by keeping the review focussed and streamlined to address its key research questions within the timeframes and allocated resource. However, with each decision to limit the scope of the study, findings of potential interest to the overall topic could have been missed. These limitations have been discussed in section 2.5 and in section 4.3 to inform possible future research opportunities.
2.3. Literature search and screening
A targeted search for academic literature was carried out in conjunction with the University of Birmingham Library Services, according to the search terms set out in the search protocol (Appendix A). Three academic databases were searched: Medline (which includes PubMed literature), CAB Abstracts, and ProQuest’s Consumer Health Database. Literature was also gathered through the following methods:
-
Purposive searches of legislation, government department and international government agencies websites, and other grey literature related to food safety/health using the search terms in Appendix A
-
Academic advisors and a call for evidence amongst our expert panel
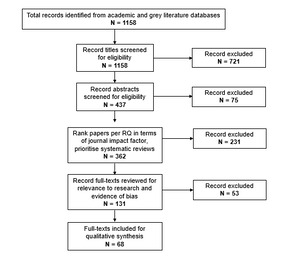
Although Food Science and Technology Abstracts was originally included in the search protocol, it was ultimately not used due to functional limitations that prevented advanced searching comparable to that carried out in the other databases. Given the substantial number of relevant records retrieved, the final search was deemed sufficiently comprehensive. In total, the search resulted in a longlist of 1158 articles which were rigorously screened as detailed in Figure 1 below.
An iterative approach to screening was undertaken. Title screening was conducted based on first level inclusion/exclusion criteria outlined in the search protocol (see Table 5 in Appendix A), followed by abstract screening of included texts. At this stage, 362 articles were deemed to be relevant to the research questions and within scope. To help shortlist further, the studies were ranked in terms of the impact factor of their journal and approximately 25-30 papers with the highest impact factor were selected for further screening for each research question. See Section 2.4 for discussion of the limitations of using impact factors.
The articles were then screened for quality using a holistic approach to determine robustness and risk of bias based on the type of methodology used. For each study, one reviewer identified the source of funding, conflicts of interest declared by the author/s, type of methodology and five common sources of bias. The results were then reviewed in a workshop with the full research team including expert advisors. At the end of the workshop, a collective decision was made to exclude narrative reviews from the final shortlist as they often reflect an unbalanced opinion and typically do not use a robust search or screening method to gather literature. Instead, it was decided that systematic reviews from the abstract screening stage would be prioritised and included in the final shortlist.
At the final stage of screening, the full length text of the papers was reviewed for relevance to the research questions and any articles that did not directly address the research questions of this study were removed. For more information on the limitations of using subjective quality assessment of the articles instead of statistical assessment or a numerical scoring guide, see Section 2.4. At each stage of screening at least two reviewers were involved and any discrepancies were resolved through discussion and consensus development. This resulted in a shortlist of 68 articles. Out of the articles reviewed, 14 were relevant to research question 1 (RQ1), 25 were relevant to RQ2, 12 were relevant to RQ3, 23 were relevant to RQ4, and 20 were relevant to RQ5.
2.4. Data extraction and reporting
Findings from the literature review were summarised according to the five research questions. Using the populated data extraction spreadsheet, the extracted data were analysed to provide a narrative synthesis of findings.
At the end of the synthesis period, we held a workshop with the FSA and expert panel to present emerging findings. Experts provided feedback on the draft findings and identified gaps and potential areas for future research. At this workshop, we also discussed differences in interpretation and methodologies within studies containing conflicting evidence and literature at risk of bias, ensuring the inclusion of the most robust evidence.
Based on the synthesised findings, the final research report was produced in collaboration with our advisors, the FSA project team and the expert panel.
2.5. Limitations
One key limitation of this study was that it did not comprehensively include all the literature available on this topic. This was because we were guided by the rapid evidence assessment methodology to deliver the report within a limited time frame and resource allocation. The scope was agreed with the FSA team and expert panel to ensure that the search strategy and screening process delivered the most relevant articles for the review. In addition, because of the project constraints, we had to modify our approach to screening to exclude some articles that may have been relevant to the research. We worked closely with the FSA project team as well as our expert advisors at each stage of the screening process to ensure consensus for each decision. We also documented our process and detailed our methodology (in section 2.3) for transparency.
A potential source of bias in this review is the use of impact factors as an exclusion factor. Impact factors were used to rank papers during screening; therefore, a number of papers were not included as they were published in journals which had a low impact factor. This can mean less visible research is overlooked, such as research papers published in niche journals.
Another source of potential bias is the exclusion of findings from animal studies. While this helped keep the review focussed on human health, it was not able to draw on findings that could be generalised from animal models to humans. This can mean some of the mechanistic understanding of nitrate and nitrite ADME and links to disease morphologies may have been omitted in this research.
We also acknowledge that this review relied on a subjective quality assessment of the articles. Some might consider this a limitation and prefer to use standard quality assessment tools such as AMSTAR or GRADE that rate each article based on a series of quality criteria. However, such frameworks always rely on a numerical scoring guide which is not always appropriate. As acknowledged by the Joint COT and COC Synthesis and Integration of Epidemiological and Toxicological Evidence (SETE) subgroup, there is no single quality assessment framework that is considered the ‘gold standard’ (Committee of Toxicity and Carcinogenicity, 2021). Hence, we agreed with the FSA project team to use a more subjective approach that holistically studied different sources of bias across the literature database and took an informed decision on that basis.
3. Results
3.1. ADME of the food additives nitrates and nitrites and the associated adverse effects and benefits
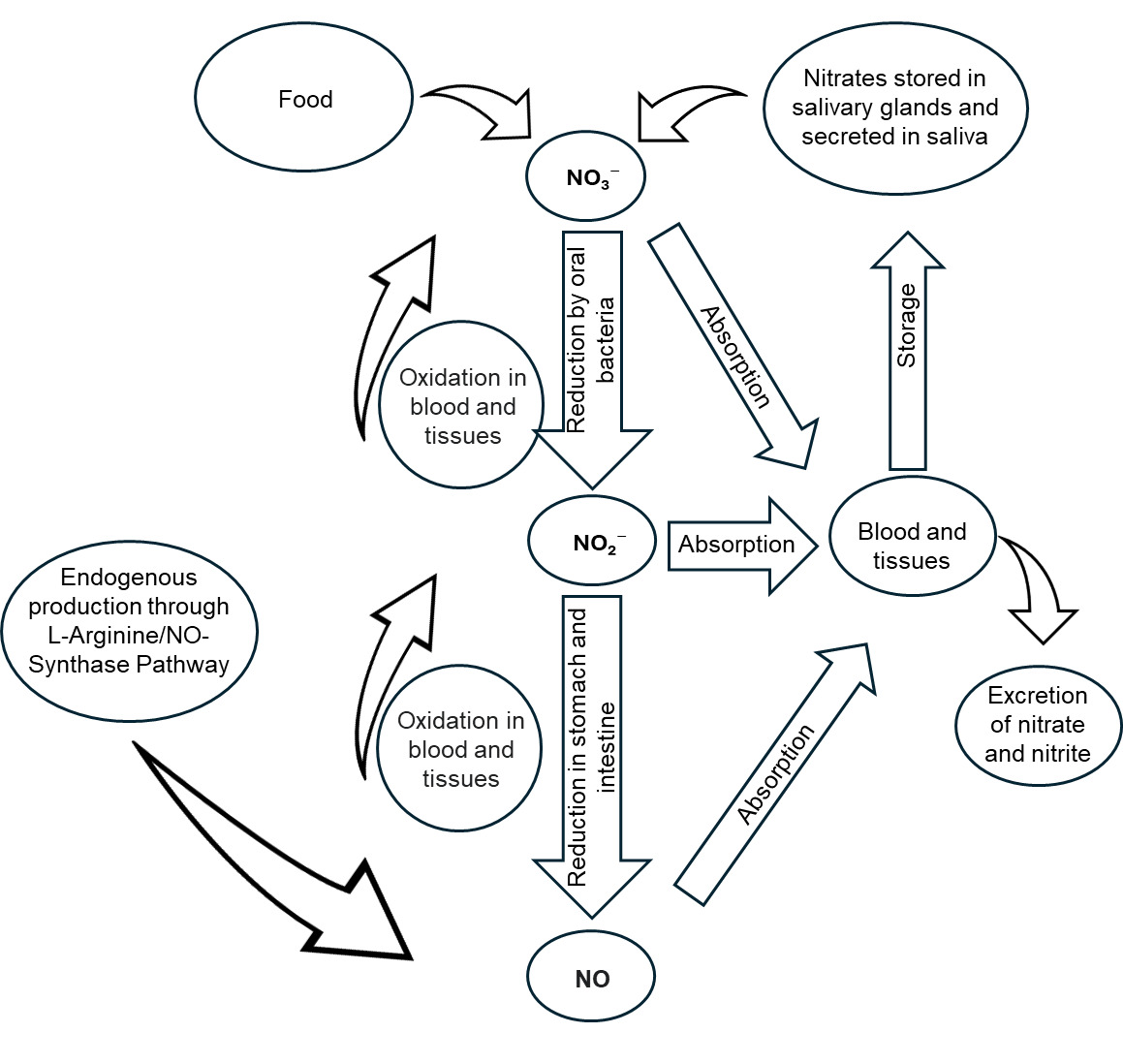
This section summarises how dietary nitrates and nitrites are rapidly absorbed, distributed systemically, metabolised into nitrite (in the case of nitrate), nitric oxide (NO) and NOCs, and excreted in urine. Unless otherwise specified, references to nitrate or nitrite in this section reflect the terminology used by the original authors, who did not always indicate whether sodium or potassium salt was used.
Figure 2 below shows a simplified scheme of nitrite and NO metabolism in the body.
Absorption
This section provides a summary of the absorption of nitrates and nitrites in the literature under normal conditions.
Nitrite
In 2017, the EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel) evaluated the safety of nitrites. In their review, the ESFA ANS Panel noted that some nitrite is formed in the upper part of the stomach, contributing to systemic nitrite concentrations (Mortensen et al., 2017a). Nitrite has a systemic availability of ~100%, and an absolute bioavailability of ~100%, meaning that nearly all orally ingested nitrite is absorbed into the bloodstream (Mortensen et al., 2017a).
Nitrate
In 2017, the ESFA ANS Panel also evaluated the safety of nitrates. The EFSA Panel denoted that nitrate from food is taken up into the bloodstream through the gastrointestinal (GI) tract and, in humans, nitrate is systemically available to 100% (Mortensen et al., 2017b).
Using a medium simulating cooked and cured meat under formulation and digestion conditions, Sirvins et al. (2024) found that nitrate absorption is influenced by the reactivity of flavonoids and ascorbate with nitrite. The results indicate that introducing phenolic compounds either as natural ingredients in cured meat or through the diet might be an efficient way to manage N-nitrosamine formation during cured meat processing, storage, and digestion (this is explored further in section 3.5).
Distribution
This section summarises the literature findings on how nitrite and nitrate circulate throughout the body once absorbed.
Nitrite
The EFSA ANS Panel summarised that nitrite is distributed throughout the body via the bloodstream and the volume of distribution was calculated by the Panel based on a few studies to be 93 L (64–124 L), indicating that nitrite is found in higher concentrations in certain tissues, compared to the blood. The Panel also noted that the conversion of nitrate to nitrite in the saliva is the most common source of nitrite for humans (Mortensen et al., 2017a).
Nitrate
The EFSA Panel summarised that absorbed nitrate circulates in the bloodstream, with about 25% taken up by salivary glands and excreted into saliva. The Panel also noted that the volume of nitrate distribution is smaller than the body water and higher than the blood volume, indicating that nitrate is distributed throughout the body. In addition, nitrate can be found in the milk of lactating women where the concentration is similar to that in the plasma (Mortensen et al., 2017b).
Metabolism
This section summarises the literature findings on the key metabolic pathways of nitrates and nitrites, including oral bacterial reduction to nitrite and gastric conversion.
Nitrite
The EFSA Panel summarised that nitrite is converted mainly back to nitrate, with minor pathways leading to nitric oxide (NO), reactive oxygen species, and protein nitrosylation. No significant first-pass metabolism occurs in the liver or intestines, meaning that systemic circulation is the main site of nitrite transformation (Mortensen et al., 2017a).
Using a dynamic gastrointestinal digester for in vitro digestions of dry-cured sausages formulated with various rates of added nitrite and nitrate, Keuleyan et al. (2022) found that during gastric digestion, residual nitrite concentrations increased in all nitrite- and nitrate-cured sausages, reaching values similar to those in the original products. No residual nitrite was detected in sausages that did not contain added nitrites. The amount of residual nitrite increased proportionally to the amount initially added, indicating a dose-dependent effect during digestion. These results contradicted the previous research cited in this paper by Kim and Hur (2018) who observed a significant fall in residual nitrite concentrations during digestion.
A dynamic in vitro digestion study of cooked and recooked meats prepared with various levels of sodium nitrite by Bonifacie et al. (2024) observed that, under simulated digestive conditions, the gut environment drives endogenous nitrosation and nitrosylation, even in the absence of nitrite added during the manufacture of products. The authors noted that digestion conditions appear to favour lipid oxidation, in particular when no nitrite is added, and the formation of potentially mutagenic compounds, such as aldehydes. Only recooked meats with the highest added nitrite (120 ppm) contained residual nitrite in the gastric compartment (Bonifacie et al., 2024).
Another experimental study using a dynamic artificial digestive system in association with a mathematical model of chemical reaction kinetics by de La Pomélie et al. (2018) observed that when nitrite interacts with haem iron during digestion, nitroso-myoglobin is formed under specific pH conditions, with lower levels observed at extreme pH (pH 7.2 and pH < 4.7). The results indicate that nitrosylation reactions are pH-dependent and influenced by myoglobin oxidation state.
Furthermore, the follow up study by de La Pomélie et al. (2019) demonstrated that that both low nitrosylation in the digestive tract and ammonia oxidation occur even without added nitrites. Adding nitrite significantly increases haem iron nitrosylation, while prior meat cooking reduces soluble myoglobin levels, limiting nitrosylation.
Nitrate
The EFSA Panel noted that in the mouth, nitrate is secreted into the saliva (20-25% of the dose), which is metabolised to nitrite by mouth bacteria (Mortensen et al., 2017b).
An experimental study by Keuleyan et al. (2022) found that baseline nitrate concentrations were detected even in dry-cured sausage products without added nitrates, suggesting that nitrate naturally occurs in meat due to the presence of nitrogenous compounds. Throughout gastric digestion, nitrate concentrations remained relatively stable, with no significant changes observed. Additionally, products formulated with higher initial nitrate concentrations retained higher residual nitrate concentrations after digestion, following a dose-dependent pattern.
Furthermore, Bonifacie et al. (2024) observed that residual nitrate concentrations were lower after a 40-minute digestion in cooked ham, but higher in recooked ham compared to original product concentrations. This is likely due to the oxidation of NO formed during NOCs degradation. In recooked hams without added sodium nitrite, residual nitrate concentrations increased and were nearly the same as those in hams that originally contained 120 ppm sodium nitrite.
Excretion
This section summarises the literature findings on how the human body excretes nitrates and nitrites.
Bonifacie et al. (2024) observed that both residual nitrite and nitrate for cooked and recooked ham were still present at the end of ileal digestion, suggesting that some untransformed compounds may reach the lower intestine.
Nitrite
Renal excretion plays a minor role in nitrite excretion, with only 0.02% of a nitrite dose found in the urine based on nitrate studies (Mortensen et al., 2017a). The EFSA ANS Panel also found no experimental studies directly assessing nitrite excretion in urine after nitrite administration.
Nitrate
The EFSA ANS Panel noted that excretion of nitrate in urine varies between 50% and 100% between studies, with the Panel noting most reliable studies showing 100% (Mortensen et al., 2017b).
Nitrosamine Formation
The following section discusses the literature findings on how nitrite from food (or nitrate converted to nitrite) can be a precursor to endogenous nitrosamine formation under acidic gastric conditions.
Nitrosamines, which are considered probable human carcinogens (IARC, 2010) and can be found in various products like food, cosmetics, and even some medications, are chemical compounds, formed from reactions between nitrates or nitrites and amines. The acidic environment of the stomach, with a low pH, promotes the conversion of nitrite to reactive nitrous acid, which can then react with amines and amides to form nitrosamines. Carcinogenesis is primarily linked to the nitrosation of aliphatic and aromatic amines, forming carcinogenic nitrosamines (Karwowska & Kononiuk, 2020).
The EFSA Scientific Opinion (Mortensen et al., 2017a) noted that NOCs, including nitrosamines and nitrosamides, are primarily formed in the upper GI tract. This formation is driven by the stomach’s pH and nitrite concentration.
An experimental study using an in vitro gastro-intestinal model by de La Pomélie et al. (2018) demonstrated that up to 20% of myoglobin can be nitrosylated under gastric conditions, supporting the idea that meat consumption may contribute to endogenous nitrosamine formation.
Keuleyan et al. (2022) found that despite the absence of nitrosamines in some products before digestion, significant endogenous synthesis of non-volatile nitrosamines occurs during gastric digestion. Approximately 25% of nitrosamines are formed during digestion.
Another experimental in vitro study by Sirvins et al. (2024) demonstrated that nitrosation reactions occur rapidly at a pH of 2.5 (gastric digestion conditions in the stomach). However, these reactions may also undergo denitrosation, or breakdown of nitrosamines, before absorption under more acidic conditions.
Bonifacie et al. (2024) found that recooked hams and higher nitrite concentrations result in increased formation of NOCs. Their study emphasized that the digestion of cooked and recooked cured meat without added nitrite highlighted a basal endogenous N-nitrosation.
A kinetic study using UV-visible spectroscopy by González-Jiménez et al. (2023) explored the reaction of sodium nitrite with dopamine and serotonin (neurotransmitters released into the gastric juice). This study found that both serotonin and dopamine can be nitrosated by nitrite via electrophilic aromatic substitution, facilitated by acidic conditions, producing stable NOCs. These results reveal that nitrite can alter biologically important molecules in the stomach and highlight the need for further research on potentially toxic effects they may have once nitrosated.
Microbiome
The oral and gut microbiomes are complex ecosystems of microorganisms, including bacteria, fungi, and viruses, which reside in the mouth and gut, respectively, and play crucial roles in overall health.
The following section summarises how dietary nitrates and nitrites interact bidirectionally with the oral and gut microbiome. Microbes reduce nitrates to nitrites (influencing systemic exposure and nitrosamine formation), while nitrite exposure in turn alters microbial composition and metabolism activity, affecting gut health and metabolite production.
Oral Microbiome
An observational study by Gonzalez et al. (2016) found that individuals suffering from migraines had higher abundances of nitrite, nitrate and NO reductase genes in their oral microbiome compared to the microbiome of those without migraines.
An experimental study using an in vitro digestion model by Niklas et al. (2023) found that nitrite from saliva contributes significantly to nitrosamine formation in digestion, supporting the idea that the body naturally forms them (endogenous nitrosation).
The EFSA re-evaluation (Mortensen et al., 2017b) acknowledged that the conversion of nitrate to nitrite is influenced by the oral microbiota, which varies among individuals. This variability affects the proportion of nitrate reduced to nitrite in the mouth, thereby influencing systemic exposure to nitrite and the subsequent formation of NOCs.
Koopman (2016) conducted an experimental study using a multiplaque artificial mouth (MAM) biofilm model. The study showed that nitrate supplementation influences microbial composition and biochemistry. Specifically, the concentration of butyrate significantly decreased in time in both control and nitrate groups, with a more pronounced decrease in the nitrate group. The production of lactate after sucrose addition was similar across treatments and control, while phosphate levels increased significantly in the control group compared to the nitrate group. These results suggest that nitrate alters microbial function, possibly suppressing beneficial fermentation as shown by reduced butyrate and modifying nutrient cycling reflected in phosphate differences. Together, these results indicate a less favourable gut microbial environment in the nitrate-exposed condition.
Gut Microbiome
An observational study by Gonzalez et al. (2016) found that migraine sufferers had increased abundances of nitrate in their gut microbiome (derived from stool samples), similar to their findings on the oral microbiome.
Gonza et al. (2024) conducted an experimental study using the in vitro multi-compartment Simulator of the Human Intestinal Microbial Ecosystem (SHIME) model. The study found that sodium nitrite exposure alters the composition and metabolic activity of the gut microbiota. Notably, the gut microbiota of patients with Inflammatory Bowel Disease (IBD), both in remission and active states, was more affected by sodium nitrite exposure compared to healthy individuals.
Nissen et al. (2023) performed a control study using an in vitro model simulating the colon. The study found that the presence of nitrites in the control group appears to enhance microbial fermentation efficiency, leading to higher production of Short-Chain Fatty Acids (SCFA) and Medium-Chain Fatty Acids (MCFA), which are beneficial for gut health. Alternative formulations without nitrites also supported beneficial fermentation, but with varying efficiency. The results indicate that microbial metabolism adapts based on the available nitrogen sources.
Bias and uncertainty
Consistency of findings
The consistency of findings within the literature included in this review varies. While some studies agree on the chemical reactivity and stability of nitrites and nitrates during digestion (Bonifacie et al., 2024; Keuleyan et al., 2022), previous reports show contradictory results (Kim & Hur, 2018). These discrepancies can be attributed to varying methodologies, such as differences in GI models, sample sizes, and experimental conditions. Inconsistencies can also arise from variations in pH levels, temperature, and dietary components.
Key sources of bias
Key sources of bias within the literature on this topic include sample selection bias, variations in experimental conditions, and potential conflicts of interest. For instance, studies using different meat products or additives may yield varying results due to intrinsic differences in their chemical compositions. Most experimental studies relied on simulated models, such as in vitro, MAM and SHIME, mimicking human physiology. While these are recognised and reliable tools, they can lack physiological complexity and host factors, for example, immune response and microbiome differences. Furthermore, individual variation in oral microbiomes can alter how much nitrate is converted to nitrite which might not be captured in these simulated models. Authors suggest that future research should focus on standardising methodologies and increasing transparency to mitigate these biases. No funding bias was identified as all studies reviewed in this section received public sector funding or none at all. Only one study declared a conflict of interest.
Uncertainties
Several uncertainties remain based on the research question on ADME of the food additives nitrates and nitrites and the associated adverse effects and benefits. The effect of confounding factors such as demographics, dietary habits, culture, genetics (including epigenetics in both human and in the microbiome), and exposure through other means beyond food additives like cosmetics or drugs is not fully understood because the majority of the studies were in vitro.
Some findings highlight the complexity of microbial responses to nitrates and nitrites, with some evidence (Koopman et al., 2016) showing a reduction in SCFA levels following nitrate exposure in oral biofilm models, while others (Nissen et al., 2023) suggest enhanced SCFA production in colonic environments with nitrite. These indirect contradictions likely reflect differences in nitrogen species, microbial niches, and experimental models, pointing to a lack of clarity about the overall impact of nitrates and nitrites across the digestive system and underscoring the need for whole-system models that track effects from mouth to colon to better understand the net impact on gut health.
Additionally, the long-term health impacts of nitrosamine formation during digestion are still unclear. Authors highlight the need for further studies to explore these variables and their potential interactions.
3.2. Health risks of nitrate and nitrite additives for human exposure
Nitrate is relatively non-toxic and, depending on the source, has been associated with beneficial physiological effects, such as lower blood pressure and enhanced exercise performance (Erichsen et al., 2024; see also Figure 4 of Karwowska & Kononiuk, 2020). However, when nitrate is converted to nitrite it can lead to potentially adverse health effects, as when ingested, nitrite can form nitrosamines, compounds linked to an increased risk of cancer (Zheng et al., 2021). Nitrite consumption is also associated with other health problems such as birth defects (Rahimi Kakavandi et al., 2021), diabetes (Nguyen et al., 2023; Srour et al., 2023) and methaemoglobinaemia (O’Neill et al., 2021; see also Figure 4 of Karwowska & Kononiuk, 2020).
The following sections detail the findings for cancer risks associated with nitrates, nitrites and processed meat, the other associated health risks identified through the review, as well as the bias and uncertainty within the papers reviewed.
Cancer risks associated with nitrates
In 2017, the EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel) evaluated the safety of nitrates as food additives, considering their potential to form carcinogenic nitrosamines and their effects on human health. In their review of nitrates, the Panel were unable to find sufficient evidence of an association between nitrates and cancer risks (Mortensen et al., 2017a). However, more recent papers have been able to identify associations between nitrate intake and the risk of breast cancer and thyroid cancer.
For instance, in their study which aimed to investigate the relationship between nitrate intakes and the risk of cancer, Chazelas et al. (2021) found that, compared with non-consumers, high consumers of nitrates as a food additive had a 24% higher risk of breast cancer [hazard ratio (HR)=1.24 (95% confidence interval (CI): 1.03, 1.48), p=0.02], especially for potassium nitrate (E 252). These associations were more specifically observed among pre-menopausal women for food additive nitrates and potassium nitrate (E 252). This study was based on data from the French NutriNet-Santé cohort and exposure to nitrates was evaluated using repeated 24-hour dietary records. In their model, the authors corrected for dietary factors such as sugar, saturated fatty acids and alcohol consumption, lifestyle factors such as physical activity, as well as family history of cancer.
Said Abasse et al. (2022) were unable to find a similar association between nitrate intake and breast cancer in their systematic review. However, in their meta-analysis, they did find a relationship between nitrate intake and thyroid cancer. When comparing the highest to lowest categories of intake, they found that high nitrate intake was associated with a 40% increase in the risk of thyroid cancer [odds ratio (OR)=1.40 (95% CI: 1.02, 1.77)]. Their meta-analysis was based on data from 41 articles, however, only three articles were related to thyroid cancer and none of these used dietary data from the UK. Furthermore, the authors were unable to correct for any confounding variables due to lack of available data.
Espejo-Herrera et al. (2016) found a positive association between colorectal cancer risk and long-term exposure to nitrate in drinking water and found that this was particularly the case for those with high processed meat intake. Individuals ingesting between 5 and 10 mg/day of nitrate from drinking water had 20% higher colorectal cancer risk [OR=1.20 (95% CI: 0.90, 1.58)], while those ingesting more than 10 mg/day had 41% higher risk [OR=1.41 (95% CI: 1.04, 1.91)] than the control group. This was a large case-control study based on hospital-based incident cases in Spain and Italy and dietary intake was estimated through food frequency questionnaires (FFQs) and published food composition databases. The authors were able to correct for variables including lifestyle factors, history of colorectal cancer in first degree relatives and other dietary factors such as intake of energy and fibre.
In a case-control cancer study conducted in Spain, Espejo-Herrera et al. (2016) found that, compared to those with the lowest intake of waterborne nitrate and processed meat, those with the highest intake of both waterborne nitrate and processed meat had a 64% higher chance of having breast cancer [adjusted OR=1.64 (95% CI: 1.08, 2.49)].
In the EFSA ANS Panel review on the safety of nitrites as food additives (Mortensen et al., 2017b), one of the papers evaluated found a relationship between nitrate plus processed meat intake and colorectal cancer.
Cancer risks associated with nitrites
Similar to their review on nitrates, in their 2017 review on the safety of nitrites as food additives, the EFSA ANS Panel was unable to find sufficient evidence of an association between nitrite intake and most cancers discussed. However, the panel did find some evidence to link nitrite from processed meat intake to colon cancer and gastric cancer (Mortensen et al., 2017b). None of the other papers reviewed found a relationship between nitrite intake and gastric cancer.
Jones et al. (2019) were unable to find a relationship between processed meat or nitrite intake and colorectal cancer, based on their analysis of data from the Iowa Women’s Health Study cohort, even when adjusting for confounding factors. Similarly, both Said Abasse et al. (2022) and Chazelas et al. (2021) were unable to identify a relationship between nitrite intake and colorectal cancer.
Another study which utilised data from the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study found that higher nitrite intake from processed red meat was positively associated with localised breast cancer, even when correcting for factors such as body mass index (BMI) and family history of breast cancer (Inoue-Choi et al., 2015). It found that those in the highest quintile of processed meat intake had a 27% higher risk of localised breast cancer compared to those in the lowest quintile [HR=1.27 (95% CI: 1.13, 1.44)]. Processed meat intake was assessed using FFQs. However, Said Abasse et al. (2022) were unable to find a similar relationship between nitrite intake and breast cancer through meta-analysis or meta-regression.
Said Abasse et al. (2022) were able to identify a relationship between dietary nitrite and both bladder and stomach cancer. Through meta-regression, they found that the risk of bladder (t=1.99, p=0.056, adjusted R2=33.77%) and stomach cancer (t=4.09, p<0.001, adjusted R2=74.06%) were positively associated with the dosage of dietary nitrite. Barry et al. (2020) were also able to find a similar relationship between bladder cancer and nitrite intake from processed meat. Using data from the New England Bladder Cancer Study, they found that those in the highest quintile of nitrite intake from processed meat had a 50% higher risk of bladder cancer compared to those in the lowest quintile [OR=1.5 (95% CI: 1.0, 2.1), p-trend=0.04], even when correcting for confounding factors such as whether an individual was in a high-risk occupation. Nitrite intake was assessed using FFQs.
Finally, Chazelas et al. (2021) found that, compared with non-consumers, high consumers of food additive nitrites and specifically sodium nitrite (E 250) had a 58% higher risk of first incident prostate cancer [HR=1.58 (95% CI: 1.14, 2.18), p=0.008].
Other associated health risks
Diabetes
Srour et al. (2023) used data from the French NutriNet-Santé cohort to investigate the association between dietary exposure to nitrates and nitrites and the risk of type 2 diabetes. They found that participants with higher exposure to additives-originated nitrites had a higher risk of type 2 diabetes compared with those who were not exposed to additives-originated nitrites [HR=1.53 (95% CI: 1.24, 1.88), p-trend<0.001]. Specifically, they found that those with higher exposure to sodium nitrite (E 250) had a higher risk of type 2 diabetes [HR=1.54 (95% CI: 1.26, 1.90), p-trend<0.001], while correcting for known risk factors such as lifestyle, nutrition and medical history. Furthermore, Nguyen et al. (2023) also found through a meta-analysis based on data from six papers that nitrite consumption was associated with a higher risk of diabetes. Specifically, they found that participants in the highest quintile of nitrite consumption were 61% more likely to develop diabetes than those in the lowest quintile [OR=1.61 (95% CI: 1.08, 2.39) p=0.02], and that a 1-mg per day increase in nitrite consumption caused a significant 4.8% increase in the risk of type 2 diabetes.
Birth Defects
Rahimi Kakavandi et al. (2021) conducted a systematic review and meta-analysis to evaluate the correlation between maternal intake of nitrate and the risk of birth defects and preterm birth. Their meta-analysis based on data from 10 papers found a significant relationship between maternal intake of nitrate and risk of heart defects. Results of linear dose-response meta-analysis indicated that each additional daily 0.5 mg of maternal nitrate intake increased the risk of heart defects. Non-linear dose response meta-analysis showed that maternal intake of nitrate higher than ~4 mg/day was positively associated with heart defects risk. Furthermore, as part of the quality assessment of the studies, adjustment for confounding variables was taken into account.
However, Rahimi Kakavandi et al. (2021) found no relationship between maternal intake of nitrate and preterm birth. In contrast, Vuong et al. (2016) found, based on data from the National Birth Defects Prevention Study conducted in the United States, that secondary amines in conjunction with high nitrate were associated with preterm birth during the first [Adjusted HR (AHR)=1.84 (95% CI: 1.14, 2.98)], second [AHR=1.89 (95% CI: 1.17, 3.07)] and third [AHR=2.00 (95% CI: 1.22, 3.29)] trimesters, even when correcting for confounding variables such as caloric intake and maternal health. Data on diet during pregnancy was collected through telephone interviews, with the median length of time between estimated date of delivery and the interview being 7.7 months.
Methaemoglobinaemia
O’Neill et al. (2021) detail a case report where two diners were poisoned with sodium nitrite which was mislabelled as sodium nitrate in the kitchen, and both were admitted to hospital after suffering from methaemoglobinaemia. While no other papers reviewed specifically explored the link between nitrite intake and methaemoglobinaemia, it has been acknowledged as a potential health risk by the EFSA ANS Panel (Mortensen et al., 2017a) and the Food Safety Authority of Ireland (Food Safety Authority of Ireland, 2016).
Bias and uncertainty
Consistency of findings
Based on the literature reviewed, there is a low level of certainty in the findings due to lack of consistency in results across papers. There is also a general lack of published research on the topic of health risks associated with nitrates and nitrites as food additives. Further research on this area that utilises precise information about participants’ diets may be needed to more accurately assess the health risks associated with the use of nitrates and nitrites as food additives (Said Abasse et al., 2022).
Key sources of bias
The main source of bias in the papers related to this research question was recall bias. Most observational studies reviewed used dietary surveys such as FFQs to estimate consumption data and dietary intake of nitrates and nitrites. Reliance on self-reported data can cause under or overestimation of dietary exposure to additives. Some studies used more objective measures such as interviews with trained interviewers or assessments using urinary or blood markers to validate FFQ responses (Chazelas et al., 2021; Vuong et al., 2016). This challenge with regards to accurately assessing nitrate and nitrite intake could raise problems when trying to establish a relationship between intakes and various health risks.
Samples from dietary studies used in some papers were also not always representative of the general population, leading to risk of selection bias. For example, the NutriNet-Santé study featured a participant pool that was not representative of the general population - 79% were women and nearly 70% of the cohort had post-secondary education (Chazelas et al., 2021). However, Chazelas et al. (2021) and Srour et al. (2023) did correct for factors such as sex and education level.
The voluntary nature of study participation has also led to dietary studies attracting participants exhibiting more health-conscious behaviours and by nature having lower consumption of foods containing added nitrates and nitrites (Chazelas et al., 2021). This can also make establishing relationships between nitrate and nitrite intake and health conditions more difficult as samples are skewed towards a healthier population.
Finally, all papers were funded through the public sector or required no funding and only two papers did report conflicts of interests for the authors.
Uncertainties
None of the papers reviewed utilised dietary information from UK participants or cohorts representative of the UK population. Given the variations in diet across countries, this is a limitation of the findings for this research question.
Furthermore, not all papers were able to correct for confounding variables such as age and family health history which could be potential risk factors (Said Abasse et al., 2022). Many papers were not able to correct for the nutritional content of foods containing nitrates and nitrites which also tend to be high in salt and fat, such as processed meats, which could be risk factors that are correlated with the diseases discussed including diabetes and cancer. Future research on other confounding factors such as occupation, dietary habits, and cosmetic use would help clarify how these factors influence cancer risk.
Moreover, a major source of uncertainty lies in the exposure assessment of nitrates and nitrites, as some studies (Chazelas et al., 2021; Srour et al., 2023) relied on broad assumptions about food composition without directly measuring nitrate/nitrite levels, despite their wide natural and industrial variation. This introduces significant potential for misclassification of intake, which may weaken observed associations with health outcomes.
A limitation to the interpretation of the data presented in the report is that the majority of the population of dietary studies were made up of healthy volunteers. Other health conditions, such as IBD or coeliac disease, which may influence the health outcomes of nitrate and nitrite exposure are not accounted for in our findings.
Finally, most of the studies reviewed looked at total dietary nitrate/nitrite, or intake of specific food products such as red and processed meat, as opposed to nitrate/nitrite intake specifically from food additives. This is relevant as the source of nitrate and nitrite intake can have an impact on the health risks associated. For instance, nitrate intake from plant sources has been associated with beneficial physiological effects, such as lower blood pressure and enhanced exercise performance (Erichsen et al., 2024).
3.3. Effects of cooking and processing
This section summarises information on food cooking and processing methods tested in the reviewed literature and their effects on N-nitrosamine formation. First an overview is provided of cooking methods looking at heat treatment and intensity and then second stage cooking. Thereafter, the report identifies different food processing methods and their effects on N-nitrosamine formation. This section then concludes with a brief explanation of the bias and uncertainty in the literature reviewed on this topic.
Cooking processes
Heat treatment and cooking intensity
The formation of N-nitrosamines is significantly influenced by cooking intensity and temperature. Higher cooking temperatures and longer cooking times generally increase the formation of N-nitrosamines. For instance, bacon fried at higher temperatures showed increased levels of N-nitrosamines, particularly N-nitrosodimethylamine (NDMA) and N-nitrosopyrrolidine (NPYR) (Sallan et al., 2020). The study by Sallan et al. (2020) investigated the effects of nitrite levels contained in foods, sodium ascorbate, and starter cultures on nitrosamine formation in heat-treated sucuk, a type of semi-dry fermented Turkish sausage. The meat was trimmed of visible fat and connective tissue, stored at -20°C, and then mixed with spices and additives, including salt, garlic, sucrose, red pepper, black pepper, cumin, and allspice. Fermentation was initiated with the addition of starter cultures, followed by gradual heat treatment in a steam cooking chamber to achieve an internal temperature of 68°C. The sausages were then dried under controlled conditions for three days at 16°C. The study found that NDMA levels increased with higher nitrite levels and cooking intensity, with the highest levels found in very well-done samples. Specifically, NDMA levels were highest in samples with the highest level of nitrite tested at 150 mg/kg nitrite. Similarly, NPYR levels increased with the use of starter cultures and higher nitrite levels, with the highest levels observed in very well-done samples. Sodium ascorbate reduced NPYR levels significantly.
Deng et al. (2021) further explored the impact of different dry-frying temperatures on nitrite levels and N-nitrosamine formation in bacon. The streaky bacon was prepared from belly pieces of Jinluo pigs. The bacon was injected with brine containing salt and varying concentrations of sodium nitrite, marinated for 20 hours at 4°C, heat-dried at 50°C for one hour, and smoked in a chamber at 50–55°C for three hours. The study found that bacon slices pan-fried at 250°C exhibited significantly larger cooking losses of nitrite compared to those pan-fried at 100°C. Residual nitrite content initially increased and then decreased sharply with higher frying temperatures. For example, the highest concentrations of residual nitrite in the samples with 0, 50, and 150 mg/kg sodium nitrite added were 9.03, 16.67, and 95.14 mg/kg at 150°C, respectively. The formation of N-nitrosamines, such as N-nitrosomethylphenylamine (NMPhA) and N-nitrosomorpholine (NMOR), increased with higher nitrite levels and frying temperatures, with the highest levels observed at 200°C and 150°C, respectively (Deng et al., 2021).
Additionally, Niklas et al. (2023) examined the levels of nitrate, nitrite, and nitrosamines in model sausages during heat treatment. Model sausages were chopped into pieces and roasted in an oven at 160°C for 30 minutes. The sausages were weighed before and after heat treatment to estimate weight loss, cooled, and stored at 4°C until analysis. The study found that nitrite concentrations decreased during processing for recipes with the highest nitrite additions, from 137–142 mg/kg in batter to 113–118 mg/kg after cooking at 80°C for 30 minutes, and further to 38–41 mg/kg after roasting at 160°C for 30 minutes. NDMA was formed during sausage production (0.2–0.3 μg/kg) and roasting (0.5–0.6 μg/kg), likely due to increased nitrosation from heat treatment. NPYR levels did not increase with nitrite addition but did increase upon heat treatment, roughly to the same extent as reported by Li et al. (2012), who found additional formation of NPYR in sausages during pan frying or deep frying at 150°C.
The EFSA Scientific Opinion (Mortensen et al., 2017a) indicated that further thermal treatment of meat products might result in an increase in volatile nitrosamines such as N-nitrosopiperidine (NPIP) and non-volatile nitrosamines like N-nitroso-2-methylthiazolidine 4-carboxylic acid, particularly at temperatures above 120°C. To reduce the formation of these compounds, it is suggested to cook meat products at lower temperatures, ideally below 70°C.
It should be noted that current FSA advice is to ensure that food is fully cooked, i.e. the middle of the food should reach a temperature of 70°C for 2 minutes or the following temperature-time combinations:
-
60°C for 45 minutes
-
65°C for 10 minutes
-
70°C for 2 minutes
-
75°C for 30 seconds
-
80°C for 6 seconds
de La Pomélie et al. (2019) conducted a study using a dynamic artificial digestive system which revealed that cooking meat significantly impacts nitrate concentrations. Specifically, the study found that cooking at higher temperatures, such as 90°C, resulted in a pronounced decrease in soluble myoglobin and an increase in the release of iron from haem. While nitrite levels were very low in meat cooked at 90°C, nitrate concentrations significantly decreased with cooking. This reduction in nitrate concentrations suggests that thermal treatment can reduce the availability of nitrates, which are precursors to nitrosamines. Additionally, the study observed that prior cooking significantly decreased endogenous nitrosylation levels, with the most pronounced effect seen between raw meat and meat cooked at 60°C, showing an overall decrease of 26.2%. Increasing cooking temperatures beyond 60°C had a lesser effect, with only a 16.4% decrease in nitrosylation levels between samples cooked at 60°C and 90°C. When comparing samples cooked at 90°C to raw meat, an overall decrease of 38.2% in nitrosylation levels was observed. These findings indicate that cooking meat at higher temperatures can mitigate the risk associated with nitrosylation during GI transit.
Second cooking stage
A second cooking stage can significantly increase non-volatile nitrosamine content. Bonifacie et al. (2021) investigated the impact of a second cooking stage on nitrosation reactions in cured and cooked meat. The method used in this study involved mixing porcine shoulder with brine containing sodium nitrite and dextrose, storing it under vacuum at 4°C, and subjecting it to a second cooking stage at various temperatures (180°C for 7 minutes and 260°C for 4 minutes). Nitrite and nitrate were extracted using the Griess reaction, and residual nitrite and nitrate concentrations were measured. The study found that there was a significant increase in non-volatile nitrosamines after the second cooking stage, particularly at moderate temperatures. For instance, the nitrosothiol content showed a significant increase with the inclusion of sodium nitrite. Nitrate concentrations increased significantly with the addition of sodium nitrite, with residual nitrate concentrations being higher than those generally cited in the literature for fresh meat. However, the presence of ascorbate reduced these levels, indicating its protective effect against nitrosation by forming intermediates that prevent nitrosation reactions.
Bonifacie et al. (2021) further investigated the chemical reactivity of nitrite and ascorbate in a cured and cooked meat model, demonstrating that a second cooking stage significantly increased the non-volatile nitrosamine content. The study showed that nitrosamines increased markedly between 0 and 40 ppm of added sodium nitrite, with additional increases not being significant beyond this point. Nitrosothiol content increased significantly with sodium nitrite addition, particularly in the absence of ascorbate. The presence of ascorbate reduced nitrosamine levels, underscoring its protective effect against nitrosation.
Food processing
Cured and uncured processing
Bak et al. (2025) also examined the levels of residual nitrite and nitrate in organic uncured ham and salami products over a period of one week. The analysis considered both opened and unopened packages during the storage period. The aim of the research was to evaluate the quality and safety of different types of ham and salami products (conventional cured, organic cured, and organic uncured) by analysing residual nitrite and nitrate, volatile N-nitrosamines, microbial load, surface colour, water activity, and pH over one week of refrigerated storage. The study found that uncured samples contained lower levels of residual nitrite and nitrate compared to cured samples. For instance, residual nitrite levels in organic uncured ham were below detection levels on day 0 and day 7 in opened samples, whereas a small amount of nitrite was detected in unopened samples. The residual levels of nitrite and nitrate in organic, uncured salami were comparable to those in cured salami, likely due to the addition of herbs and spices and the reduction of nitrate by nitrate reductases from microorganisms. However, the study highlighted that consuming only uncured meat products may not eliminate the intake of nitrite and nitrate due to natural sources of nitrate in some products.
Plant polyphenols and flavonoids
Plant polyphenols in fruits, vegetables, and plant foods can be used as alternatives to nitrate and nitrite food additives to reduce the usage of nitrite additives and inhibit production of harmful nitrosamines. Deng et al. (2022) found that apple polyphenol reduced protein oxidation and NMPhA content, while tea and cinnamon polyphenols at high concentrations decreased NMPhA levels by 38.87% and 23.09%, respectively. Moreover, van Breda (2021) supports the notion that incorporating natural antioxidants and bioactive molecules into meat processing can improve food safety by reducing the formation of harmful compounds. For further information on plant polyphenols see section 3.5 on alternatives to nitrates and nitrites.
Sirvins et al. (2024) research focused on limiting the formation of N-nitrosamines in meat processing. Using N-acetyltryptophan as a secondary amine target, the study found that flavonoids such as epicatechin, rutin, and quercetin were effective in reducing the formation of N-acetyl-N-nitrosotryptophan (NO-AcTrp) at both pH 2.5 and pH 5, with epicatechin being two to three-fold more efficient. The researchers identified common mechanistic pathways involving flavonoid oxidation by nitrite, followed by C-nitration and covalent coupling between NO-AcTrp and flavonoids or their nitro and nitroso counterparts. These findings suggest that flavonoids could play a significant role in managing N-nitrosamine formation during meat processing, storage, and digestion, thereby enhancing food safety and reducing health risks associated with cured meat consumption.
Ascorbic acid
Ascorbic acid, a powerful antioxidant (although it can act as a pro-oxidant under some conditions, however unlikely to occur during standard good processing practices), affects nitrite levels and nitrosamine formation in food processing. Bonifacie et al. (2021) showed that ascorbate inhibits nitrosation in cured meat by forming intermediates. The presence of ascorbate reduced nitrosothiol levels, especially at moderate temperatures. Deng et al. (2022) found ascorbate could both slow and speed up nitrosamine formation but effectively reduced NMPhA content in dry-fried bacon. Ascorbic acid thus has the potential to protect against nitrosamine formation. Additionally, Sallan et al. (2020) identified that sodium ascorbate had a complex effect, sometimes increasing NDMA levels while reducing NPYR levels. For further information on ascorbic acid see section 3.5 on alternatives to nitrates and nitrites.
Moreover, the EFSA Scientific Opinion identified that incorporating antioxidants such as ascorbic acid, ascorbyl palmitate, or erythorbic acid in meat formulations can reduce nitrosamine levels, except for volatile nitrosamines like N-nitrososarcosine, NDMA, NPYR, and NPIP (Mortensen et al., 2017a).
Fermentation and starter cultures
Fermentation is a widely used food processing technique that can significantly influence nitrite levels. It should be noted that the strain of starter culture used can impact nitrosamine formation in fermented meat products. Sallan et al. (2020) studied how nitrite levels, sodium ascorbate, and starter cultures influence nitrosamine formation in heat-treated sucuk, a type of semi-dry fermented sausage. The study found that the use of starter cultures, such as Lactobacillus plantarum and Staphylococcus xylosus, increased nitrosamine formation, particularly NPYR and NPIP. Higher nitrite levels led to increased nitrosamine formation, with NDMA levels being highest in samples with 150 mg/kg nitrite.
Bias and uncertainty
Consistency of findings
The literature included in the review generally agrees that higher cooking temperatures and nitrite levels increase the formation of harmful NOCs and N-nitrosamines. However, discrepancies arise due to different methodologies, such as variations in cooking methods, types of meat products, and the presence of additives like antioxidants. For instance, while some studies found that ascorbate reduces nitrosamine formation, others reported complex effects depending on the type of nitrosamine. The compounds discussed in this section were identified within the reviewed literature, and do not provide a comprehensive list of all NOCs and N-nitrosamines.
Key sources of bias
Key sources of bias within the literature include variations in experimental design, sample sizes, and the types of meat products used. For example, studies using different meat types or cooking methods may yield varying results, impacting the generalisability of findings. Additionally, the presence of additives like antioxidants can introduce bias, as their effects may not be uniformly understood or applied across studies.
No funding bias or conflict of interest were identified as all studies reviewed in this section received public sector funding.
Uncertainties
Several uncertainties remain based on this research question, including the effects of confounding factors like demographics, dietary habits, culture, genetics, and exposure through other means beyond food additives, such as cosmetics or drugs. These variables can significantly impact the formation and effects of NOCs and N-nitrosamines (Bonifacie et al., 2021).
3.4. Occurrence in food where the additives are authorised or unauthorised
This section summarises information on the occurrence of nitrates and nitrites both naturally in plant-based foods, and as permitted food additives for various food categories and focuses on key sources of dietary intake and food categories that contribute to nitrate and nitrite exposure.
Food categories where nitrates and nitrites are naturally occurring and foods where additives use is extensive
Authorised use refers to groups of foods for which specific food additives, such as nitrates and nitrites, have been approved for use by regulatory authorities such as EFSA.
Findings across studies found that naturally high nitrate content from vegetables dominated overall dietary intake, mainly due to the use of fertilisers (Abd Hamid et al., 2020). The Food Safety Authority of Ireland’s (2016) report on a Total Diet Study carried out to measure dietary exposure of the Irish population to food additives found that vegetables contributed 76% and 64% to total dietary intake of nitrate for adults and children respectively. Similarly, an observational study conducted on 228 children aged 10-11 years in Granada, Spain found that vegetables accounted for over 70% of dietary nitrate intake (Hinojosa-Nogueira et al., 2023).
Erichsen et al. (2024) conducted a study exploring different sources of dietary nitrate and nitrite intakes in a cohort of 55,754 Danish citizens. The study reported that plant-based foods were responsible for 76% of nitrate intake. These foods included fruits, vegetables, legumes, wholegrains, nuts and oils. Dependent on dietary source, nitrate has been found to have beneficial physiological effects for consumers, particularly if from plant-based foods. These include a reduction in blood pressure, beneficial effects on cancer risk, and better exercise performance (Babateen et al., 2018; Hinojosa-Nogueira et al., 2023).
In a study by the National Institute for Public Health and the Environment (Netherlands) (2020) estimating the combined dietary intake of nitrates and nitrites in the Dutch population aged 1-79 years, nitrate contributed to 95% of the combined exposure from nitrate and nitrite as food additives. The highest contribution of combined exposure from food additive use is accounted for by meat products, of which heat-treated meat products including sausages, ham and paté, contributed more than non-heat-treated meat products such as dry sausages and bacon.
Across studies, nitrite exposure was found to be comparatively low due to its restricted use as an additive in processed foods. However, processed meats are major contributors to dietary intake of nitrites, as sodium and potassium nitrite are commonly used as food additives in these products (Chazelas et al., 2020; Zhong et al., 2021). The majority of studies exploring processed meat products looked at sausages, bacon, and ham. Notably, out of 13 meat products sold commercially and sampled in Italy, 10 samples contained nitrate concentrations exceeding the EU legal limits (Berardi et al., 2021). Ongoing monitoring of levels of food additives is needed for meat products to ensure food safety.
Risk assessment
The current ADIs as established by EFSA are 3.7 mg/kg bw/day for nitrate and a more conservative 0.07 mg/kg bw/day for nitrite due to its greater reactivity and carcinogenic potential (Nader et al., 2022). There is a concern for excessive intake of nitrates and nitrites in high processed meat consumers, many of which are young children. Milešević et al. (2022) investigated nitrite intake from meat products in Serbian children and found that 62.67% of children reported that they consumed meat products 2-3 times a week. These groups are more likely to exceed ADIs due to direct food additive exposure as nitrite salts are commonly used in processed meat (Chazelas et al., 2020).
For instance, Milešević et al. (2022) also found that 6.4% of the children in their study exceeded the ADI for nitrites from consumption of meat products, with a higher proportion observed among children aged 1–3 years. Similarly, a study conducted in Greece found that 6.6% of consumers exceeded the ADI of nitrite from consumption of processed meat products alone, with the highest levels of nitrite consumption (0.18 mg/kg bw/day) found to be almost four times the ADI, for children aged 0-9 years (Kotopoulou et al., 2022).
Abd Hamid et al. (2020) explored dietary exposure to nitrates and nitrites through cured meat products amongst school children aged 1-18 years in Brunei Darussalam and found that intake increased proportionally with age. The youngest age group studied (1−3 years old) had an estimated intake of 1.29 mg/day of nitrate and 0.85 mg/day of nitrite. Children aged 4-6 years old had an estimated nitrate and nitrite intake at 1.95 mg/day and 1.29 mg/day, respectively. These were approximately twice the amount consumed by children from the youngest age group.
In their re-evaluation of nitrites, EFSA (Mortensen et al., 2017a) found dietary intake to be below or equivalent to ADI levels across food groups. Mean exposure to nitrites from their use as food additives ranged from <0.01 mg/kg bw/day in infants (12 weeks-11 months), children (3-9 years), adolescents (10-17 years) and the elderly (≥ 65 years) to 0.06 mg/kg bw/day in toddlers (12-35 months) and children (3-9 years). Sausages and preserved meat were the greatest contributors to this intake.
Similarly, in their re-evaluation of nitrates, EFSA (Mortensen et al., 2017b) found dietary intake to be below ADI levels. Mean exposure to nitrates from their use as food additives ranged from 0.01 mg/kg bw/day in infants (12 weeks-11 months) to 0.24 mg/kg bw/day in toddlers (12-35 months). Meat products, including preserved meat and sausages, and cheese were the greatest contributors to this intake.
Exposure to nitrates and nitrites in dairy products varies depending on the type of product and regional dietary patterns. A study in Iran found that dietary exposure to nitrates through milk samples remained below the ADI across 10 provinces, suggesting minimal risk from milk consumption (Chamandust et al., 2016). However, Dutch studies found that the second main contributor to combined exposure was cheese, particularly gouda (National Institute for Public Health and the Environment (Netherlands), 2016).
Non-compliant use of the additives
Non-compliant use refers to groups of food where authorised additives are present at concentrations exceeding the legal thresholds (see Introduction: Comparing GB legislation with other countries).
The Rapid Alert System for Food and Feed (RASFF) is a system used in the European Union to quickly share information about food safety risks, operated by the European Commission (European Commission, n.d.). It was established so that food safety authorities in Member States can take fast action to protect public health when unsafe food or feed is found. A search was conducted for all instances of nitrate and nitrite reported in food across all member countries between 2016 and 2024. The earliest year for notifications related to nitrate was 2020, and 2022 for nitrite. Where concentration values were available for excessive additive use, they have been included in this report. Food groups where non-compliant occurrence was identified were similar across nitrates and nitrites, with meat and vegetables appearing in both.
Nitrate
There were a total of 17 notifications related to excessive nitrate in food between 2020 and 2024. Italy was the most frequent country of origin for the food products notified, whereas Czech Republic, Belgium, and Poland were the most frequent notifiers.
15 notifications were related to naturally occurring nitrate in fruit and vegetables. The majority of these notifications (12) related to excessive nitrate content in spinach, while two highlighted risks associated with excessive nitrate content in rocket. One notification from Germany alerted consumers to potential excessive nitrate intake through consuming concentrated beetroot juice from the United Kingdom/Northern Ireland.
Organic dry sausage was withdrawn from the French market after the producer found excessive potassium nitrate in the product. For traditional immersion and dry cured meat products, the maximum permitted level of added potassium nitrate is 250 mg/kg (Martínez-Pineda et al., 2021). Additionally, excessive nitrate content in organic baby food from France led to recalls of organic baby food in Belgium and organic green bean puree in France (European Commission, n.d.). Three samples of the baby food taken between October and November 2022 found nitrate concentrations between 282 and 457.8 mg/kg, which is above maximum permitted limits of 200 mg/kg.
Nitrite
There were a total of four notifications related to excessive nitrite content in food between 2022 and 2023. Italy was the most frequent country of origin for the food products notified, as well as the most frequent notifier.
Two notifications from Austria and Germany related to excessive nitrite content in natural mineral water from Turkey and baby spinach from Italy, respectively.
Two notifications related to poultry and meat products were considered potentially serious and resulted in product recalls. Excessive potassium nitrite was detected in fresh vacuum-packed sausage in Italy. A notification from France highlighted that defective nitrite salt used in cooked sausage in halal delicatessens in France posed a risk of development of sulphite-reducing Clostridia that can cause food poisoning.
Unauthorised use of the additives
Unauthorised use refers to groups of foods that contain additives not approved for use in that category, thereby raising potential health and safety concerns. Fish appeared in the results for unauthorised occurrence of both nitrate and nitrite.
Nitrate
There were two notifications in the RASFF Consumers Portal (European Commission, n.d.) concerning unauthorised occurrence of nitrate in fish and fish products, with frozen cod loins arriving in Spain from China containing unauthorised sodium nitrate at 43.4±9.1 mg/kg. Another notification from Italy also reported unauthorised sodium nitrate at levels of 11 mg/kg in defrosted tuna arriving from Spain.
Nitrite
There was one notification indicating the presence of unauthorised sodium nitrite in defrosted tuna from Spain, at 5 mg/kg.
The majority of notifications on the RASFF Consumers Portal referred to non-compliant occurrence rather than unauthorised occurrence. Over the years, there has been a general decline in notifications related to nitrate occurrence in food, with notable exceptions in 2021 and 2023, where six and five notifications were reported, respectively. In contrast, 2024 saw a significant decrease, with only two notifications recorded. There were significantly fewer notifications related to nitrite occurrence in food. The frequency of these notifications has fluctuated over the years, with three incidents reported in 2023, and just one each in 2022 and 2024.
The findings of both non-compliant and unauthorised nitrate and nitrite presence in food highlight the importance of rigorous food safety monitoring and enforcement of legal limits to ensure consumer safety for all age groups. However, it is important to note the difference between non-compliant occurrence as a result of naturally occurring nitrates and nitrites and unauthorised use of additives, as this can mislead consumers.
Bias and uncertainty
Consistency of findings
Findings were generally consistent across studies and were in line with the findings of previous research. However, variations in dietary habits and manufacturing processes led to some differences in findings. For examples, studies from Serbia (Milešević et al., 2022) and Greece (Kotopoulou et al., 2022) found that some consumers exceeded the ADI for nitrite through consumption of processed meat, whereas a study from Iran found that nitrate exposure from milk products was below the ADI (Chamandust et al., 2016). Furthermore, in their re-evaluation of nitrates and nitrites, EFSA (Mortensen et al., 2017b, 2017a) found that total dietary nitrate and nitrite intake was below or equivalent to ADIs.
Key sources of bias
One significant source of bias in the studies reviewed is sampling bias. Many studies are based on specific sample populations, such as self-selected cohort participants in studies used to measure dietary exposure in observational studies, or product types from specific regions, such as meat sampled from two regions in Southern Italy for nitrate and nitrite concentrations (Berardi et al., 2021). This limits the ability to generalise findings to other groups or products as they may not fully represent the broader population.
No conflicts of interest were reported. With the exception of one study that received no funding, the majority of studies received public sector funding.
Uncertainties
The effect of confounding factors and uncertainties on the accuracy of results was highlighted in a study by Crowe et al. (2020) on residual nitrite concentrations in bacon. The range of residual nitrite concentrations (1–56 mg/kg) for 89 samples of smoked and unsmoked bacon collected from UK supermarkets were well below the maximum permitted limit of 175 mg/kg specified by the European Commission. A study investigating residual nitrite and nitrate in cured and uncured ham and salami in Austria reported similar results (Bak et al., 2025). Although levels were below the permitted limits in the EU, the levels of nitrite and nitrate added to the products were not known. There was a source of uncertainty in both studies; it could not be determined if manufacturers were adding nitrite at authorised limits, or if sodium nitrite was rendered undetectable through reduction to NO during processing. Future research on other confounding factors such as presence of reductants, temperature, and pH would help clarify how these factors influence residual nitrate and nitrite concentrations.
3.5. Alternatives to nitrate and nitrite food additives
This section summarises information on alternative food additives and ingredients tested in the reviewed literature that share the functionality of nitrates and nitrites. First, an overview of the alternatives to nitrate and nitrite food additives is provided, detailing how they are tested in the literature. Thereafter, the report outlines the technological functionality imparted by the alternative additives and ingredients and the risks associated with these. This section then concludes with a brief explanation of the bias and uncertainty in the literature reviewed on this topic.
Overview of alternative additives and ingredients and how they are tested
Alternative additives and ingredients were derived from non-synthetic sources such as rosemary, green leafy vegetables like spinach, lettuce, chard, winter mushroom, olive, cinnamon, peppermint and green tea. Table 2 provides a summary of the additives and ingredients tested in the literature, detailing how (if at all) the alternative was treated before being added to the food, the test food sample, the extent to which nitrate or nitrite additives were used, and what technological functions it imparted to the food product.
Functionality of the alternative food additives and ingredients
In this section, we briefly describe the mechanisms through which alternatives to nitrate and nitrite food additives achieve the same functionality as nitrate and nitrite additives. We do not anticipate that we will comprehensively cover each additive specified in Table 2 but provide a brief summary of the most common mechanisms.
The aim of seeking and testing appropriate alternatives to nitrate and nitrite additives is to produce the same technological properties imparted to food products by the latter. These include preservation effects by preventing the growth of harmful microorganisms, maintain the pink-red colour of meat and add flavour, and prevent cheese from bloating during fermentation (Mortensen et al., 2017b, 2017a). However, the addition of nitrites and nitrates is associated with the formation of harmful nitrosamine compounds,as detailed in sections 3.2 and 3.3. Thus, another aim of alternatives to nitrate and nitrite food additives is to reduce the production of nitrosamines either during food processing, storage or digestion.
Many alternatives listed in Table 2 contain polyphenols, such as extracts from Prunus meme, tea, olive, apple, cinnamon, etc. Polyphenols promote the conversion of nitrite to NO under acidic conditions (such as gastric juices), which means that during digestion they help reduce the availability of residual nitrite in the food product (e.g., Deng et al., 2022; Ren et al., 2024; Sirvins et al., 2024; Totaro et al., 2024). Polyphenols can also participate in competitive nitration, reducing availability of nitrite for nitrosation reactions (e.g., Cheng et al., 2025). The nitrite scavenging property of alternatives such as polyphenols helps to reduces the nitrite substrate in the food product and during digestion, which decreases the likelihood of nitrosation of nitrite and ultimately decreases the production of harmful nitrosamines.
Polyphenols, flavonoids and other alternatives are also chosen for their anti-oxidative properties, which reduces the oxidation of protein and lipids in the food product and avoids food from degrading. This is often through the formation of NO, which can react with myoglobin (Mb) to form NO-Mb which is a stable compound and contributes to the desirable colour of the meat product, and prevent oxidation to metmyoglobin which is sign of protein oxidation (e.g., Gao et al., 2022; Zhu et al., 2020).
Other alternatives to nitrate and nitrite food additives are derived from essential oils and include active components such as carvacrol in oregano oil, cinnamaldehyde in cinnamon oil, and citral in Chinese pepper oil. These components are known to disrupt the integrity of bacterial cell membranes and lead to the leakage of cellular contents and ultimately cell death of microorganisms such as Clostridium sporogenes (e.g., Nissen et al., 2023; Pinelli et al., 2021). Other sources of antimicrobial activity include the use of natural sources of nitrate, such as leafy vegetables, radishes, beetroot etc., which inhibit the growth of various pathogenic microorganisms including Listeria monocytogenes, Clostridium sporogenes, and Clostridium perfringens (e.g., de Carvalho et al., 2024; Martínez et al., 2019).
Risks associated with the alternative additives
A majority of the literature reviewed with respect to alternatives to nitrate and nitrite additives did not mention any risks associated with the additives being tested. There was an assumption by most authors that the organic sources of nitrate were better than inorganic additives. For example, this could be because the natural alternatives to nitrate and nitrite food additives offered benefits such as reduced nitrosamines and residual nitrite in the food product.
There were a few exceptions of studies in which the authors did note potential risks or concerns, which have been elaborated below. Ren et al. (2024) studied the inhibitory effects of polyphenols on the nitrosation reaction to reduce the formation of nitrosamines with carcinogenic effects. While they noted positive effects, they also highlighted a potential risk due to the reaction between polyphenols and nitrite to form semiquinone radicals and toxic quinones. These could contribute to oxidative stress in the body. Additionally, the anti-nitrosating properties of polyphenols created NO as a by-product and the authors warned that excessive levels of this may lead to inflammation and tissue damage for consumers. The risk associated with high doses of polyphenols (0.9 g/kg) was also raised by Cheng et al. (2025) who explained that this could stimulate lipid oxidation in food systems.
Investigating the properties of S-nitrosothiols, Andrade et al. (2024) found that they produced extremely low amounts of residual nitrite in the food product, which was a positive function in terms of subsequent nitrosamine production. At the same time, the authors highlighted that the nearly absent levels of nitrite could lower the stability of the product if stored for long periods, which could cause colour instability, lipid oxidation, protein degradation and microbial activity.
Other authors acknowledged the nascent research in this area and that further research was necessary to assess safety of the use of the alternatives to nitrate and nitrite additives (de Carvalho et al., 2024; Gao et al., 2022; Shpaizer et al., 2018).
Bias and uncertainty
Consistency of findings
There were very few studies investigating the same alternatives; however, several studies tested different additives or ingredients with the same basic functional structure and mechanism. For example, multiple authors looked at the effect of polyphenols but derived them from different natural substances, including apple, cinnamon, grapeseed, tea, olive, gallic acid (Cheng et al., 2025; Gao et al., 2022; Nissen et al., 2023; Ren et al., 2024). In such cases, there was a relatively high level of consistency in findings across the literature base. Similarly, the reviewed studies did not note contradictions with results from previous studies (not included in the current report), suggesting that the emerging results on alternatives were broadly consistent. However, these findings have to be treated with caution as this area of research is in its early stages.
Sources of bias
The studies reviewed as part of this report were limited only to experimental laboratory investigations to test samples of meat prepared with and without traditional and alternative additives. However, there was no research data testing them in vivo, either in animal or human studies. The next stage of the research may be to expand the methodology used to test. Additionally, there was little methodological consistency between studies in terms of the set of criteria used to demonstrate functionality of the alternative additives, for example in terms of the NOCs generated or growth of certain microbes. Finally, the potential risks of the alternatives were not comprehensively tested or reported, which suggested an optimism bias in the literature.
Finally, all papers were funded through the public sector and only two papers reported conflicts of interests for the authors.
Uncertainties
The most fundamental uncertainty is whether the alternatives to nitrate and nitrite food additives proposed in the reviewed literature are truly ‘better’ than nitrate/nitrite additives. While most authors explained that the alternative was sourced from organic produce obtained commercially, few clarified whether the essential ingredient obtained following extensive processing was approved for use. Deng et al. (2022) presented one exception where they specifically shared that the plant polyphenols they tested were approved as food additives in meat products and other food categories. Most studies did not raise the potential issues in terms of food regulation. There was an assumption that substituting inorganic sources of nitrate and nitrites with natural sources, through the use of extracts from green leafy vegetables or beetroot etc., would be beneficial due to the reduced reliance on inorganic additives and may be preferred by consumers because of the associated ‘green’ labels. However, future research would need to test the level of nitrate and nitrite in these alternative food products, as the current regulation suggests that the addition of plant extracts to food for food additive functions will need to meet the definition of a food additive and comply with the conditions set out in the food additive legislation (as per The Standing Committee on Plants, Animals, Food and Feed, n.d.).
The potential risk of overexposure to polyphenols has been highlighted above, in terms of potential generation of toxic compounds, oxidative stress, inflammation in the host body. It is also important to note that there are many other side effects of polyphenols, and they can exert unintended harmful effects on the body such as block iron uptake, inhibit digestive enzymes or impact hormonal balance (Duda-Chodak & Tarko, 2023). These were not explored as part of this research but could be included in future research focussed on specific alternatives.
4. Discussion
4.1. Interpretation of results
ADME of nitrate and nitrite additives in food and the associated adverse effects and benefits
The literature reviewed provides evidence of how dietary nitrates and nitrites are rapidly absorbed, distributed systemically, metabolised into nitrite (in the case of nitrate), NO and NOCs, and largely excreted in urine (ADME processes). Under acidic gastric conditions, nitrite and nitrate contribute to endogenous nitrosation and nitrosylation, forming NOCs. Evidence also shows that nitrite intake can alter gut microbiome composition and fermentation, with implications for gut health. Oral bacteria drive nitrate to nitrite conversion, influencing systemic nitrite exposure and nitrosation, however, further research is needed to fully elucidate the impact of the microbiome on these processes.
Health risks of nitrate and nitrite additives for human exposure
With regard to the health risks associated with nitrates and nitrites as food additives, the findings from the papers reviewed were mixed. Some papers were able to find relationships between nitrate and nitrite consumption and various types of cancers, such as breast cancer (Chazelas et al., 2021), colorectal cancer (Espejo-Herrera, Gràcia-Lavedan, et al., 2016), and thyroid cancer (Said Abasse et al., 2022), while others were unable to find similar relationships (Jones et al., 2019). Most of this evidence is epidemiological, but it is also supported by mechanistic work. Diabetes (Srour et al., 2023), birth defects (Rahimi Kakavandi et al., 2021) and methaemoglobinaemia (O’Neill et al., 2021) were also health risks associated with nitrates and nitrites in the literature. However, this was based on the findings of a small number of human studies and it is challenging to study nitrate/nitrite intake using FFQs. Other health conditions such as IBD, coeliac disease, and other GI disorders—which may influence nitrate and nitrite metabolism or affect the oral and gut microbiome—were not accounted for in the available data and therefore not reflected in the findings. The evidence from this review provides mixed findings, and further research is needed to establish definitive links between nitrate/nitrite intake and health risks.
Effects of cooking and processing
The literature reviewed looking at cooking methods identified that higher cooking temperatures and longer cooking times generally increase the levels of harmful nitrosamines like NDMA and NPYR. With respect to the level of food processing, cured meats with higher nitrite levels form more nitrosamines, while uncured meats have lower levels but can still contribute due to naturally occurring nitrates. Plant polyphenols including flavonoids can reduce nitrosamine levels by inhibiting protein oxidation. Ascorbic acid helps prevent nitrosation by forming protective intermediates. Fermentation and starter cultures also affect nitrosamine formation. Controlling these factors can enhance food safety by minimising harmful nitrosamines.
Occurrence in food where the additives are authorised or unauthorised
Studies from various countries, including Ireland, Spain, and Denmark, consistently show that plant-based foods contribute significantly to nitrate intake. However, processed meats are also major contributors to dietary nitrite intake, sometimes leading to consumers exceeding acceptable daily intakes, particularly young children, which could pose potential health risks. Furthermore, there are cases where food products, including processed meats, have exceeded regulatory limits for nitrate and nitrite content. This emphasizes the importance of rigorous food safety monitoring and enforcement of legal limits to ensure consumer safety.
Alternatives to nitrate and nitrite food additives
Given the potential risks and harmful effects associated with the use of nitrate and nitrite additives in food, research has explored several alternatives that promise similar functionality but lower risks. Many of these alternatives to nitrate and nitrite food additives rely on bioactive ingredients in green leafy vegetables, essential oils, tea, fruits and mushrooms, that impart beneficial functional properties such as nitrite scavenging, antioxidation and antimicrobial effects. However, this review highlighted that there were inconsistencies in the criteria used to test for these properties across studies and there was not comprehensive testing of potential risks associated with the proposed alternatives.
It should be noted that most authors mentioned that the alternatives to nitrate and nitrite food additives came from commercially sourced organic produce but did not clarify if the processed ingredient was approved for use or raise potential issues related to food regulation. It was assumed that using natural sources of nitrate and nitrites from vegetables like greens or beetroot would be beneficial due to reduced inorganic additives and preferred by consumers because of ‘green’ labels. But neither of these points have been rigorously tested, revealing that these gaps in understanding of these ingredients as well as their safety assessment may need to be addressed before products using alternative additives are approved for commercial use.
These findings, however, are associated with uncertainty arising from different sources. For instance, the scope of this research was limited to human exposure to nitrites and nitrates from food additives. Therefore findings from the literature in relation to animal models were excluded but may be able to shed light on important aspects of ADME processes that could be translated from animals to humans (e.g., Chen et al., 2015). Also, exposure and risks originating from non-additives sources of nitrates and nitrites, such as organic produce or water, and the impact of using nitrate-rich sources of fertiliser and feed on food chains was not explored in this study.
None of the papers reviewed utilised dietary information from UK participants or a population representative of the UK, which may limit the generalisability of the findings to the population in the UK. Also, the levels of nitrite and nitrate added to food products was not always clear as studies sometimes only assessed the level of residual nitrite (Bak et al., 2025; Crowe et al., 2020).
Furthermore, factors such as demographics, dietary habits and culture may result in cohort-specific influences that are currently largely unexplored in the literature. Similarly, while there may be an impact of genetic differences on how the body processes nitrates and nitrites (e.g., DeBenedictis et al., 2024), the literature reviewed in this study did not capture these effects and may need to be explored in future research.
4.2. What does this mean for the consumer?
While there is literature suggesting an association between the consumption of food additives and increased risk of potential harmful effects, such as different types of cancer, diabetes and heart defects in unborn children, the data are not conclusive. The risk appears to be highest when considering diets with very high levels of processed meat that contains inorganic nitrate and nitrite compounds.
Consumers can reduce their exposure to potentially harmful nitrite-derived compounds by moderating their intake of cured and processed meats. The NHS recommend a maximum intake of 70 g of meat per day, including cured meat (NHS, 2024). This has been consistently part of UK healthy eating advice as part of the Eatwell Guide as well as being compatible with sustainable diet goals. Consumers may want to choose products with lower added nitrites or opt for plant-based alternatives and favour fresh meats over recooked ones, as long as texture and flavour are unaffected and shelf-life and product stability are maintained.
It is also suggested that consumers exercise caution when cooking processed meats containing additives. As such, there is a need for risk management regarding safe cooking practices and how to communicate this information to the wider public. This would help ensuring the public are aware that they cook products at the temperatures required to kill any pathogens and do not cook at elevated temperature and longer durations which are associated with an increase of harmful compounds, such as nitrosamines, which have been linked to carcinogenic effects.
Consumers should be aware of the trend towards green labelling or clean labelling to emphasise the use of alternative additives from natural sources that are said to be safe or better for health. The research associated with these alternatives to nitrate and nitrite food additives is in its early stages, with more comprehensive testing to be done on the potential benefits and risks of the traditional versus alternative additives to food.
4.3. Directions for future research
Future research on the safety of nitrates and nitrites as food additives should broaden its scope to account for a wider range of dietary sources beyond foods containing nitrates and nitrites as food additives. Current evidence indicates that fruits and vegetables contribute to approximately 80–95% of human dietary nitrate intake (Kotopoulou et al., 2022), with leafy greens such as spinach and cabbage being particularly rich sources (Abd Hamid et al., 2020; Blekkenhorst et al., 2017). However, other naturally occurring sources must be considered to obtain a more comprehensive understanding of total exposure, overall body burden and the cumulative risk. Drinking water, especially from groundwater sources, can contribute significantly to nitrate intake, particularly in certain geographical regions where fertiliser use is prevalent (Food Safety Authority of Ireland, 2016). This could suggest a case for a whole food system approach to nitrate content of foods.
In contrast, if the aim is to better understand the specific association between nitrates and nitrites as food additives and the health risks for humans, further research could be conducted to improve our understanding. Further research on this area that utilises precise information about participants’ diets may be needed to more accurately assess the health risks associated with the use of nitrates and nitrites as food additives (Said Abasse et al., 2022). This is relevant as the source of nitrate and nitrite intake can have an impact on the health risks associated. For instance, nitrate intake from plant sources has been associated with beneficial physiological effects, such as lower blood pressure and enhanced exercise performance (Erichsen et al., 2024).
There is an opportunity in the future to integrate in vitro and in vivo approaches to provide a comprehensive understanding of health risks and benefits of nitrite and nitrate use in food. While in vitro systems provide valuable insights into the chemical reactivity of nitrites and nitrates in the GI tract, in vivo studies are necessary to account for individual physiological variations, microbiome interactions, and metabolic pathways that influence nitrosation and potential health risks. Furthermore, investigating long-term effects on DNA adduct formation can help assess potential genotoxic risks. Dietary and microbial factors affecting nitrosamine formation should also be explored to refine risk assessments. In addition, alongside conventional methodologies, it would be valuable to consider incorporating a New Approach Methodologies (NAMS) framework. This could provide innovative and complementary tools for evaluating biological effects, exposure levels, and mechanistic pathways in a more refined manner.
Another key area for future work is the gut microbiota’s influence on nitrite metabolism and NOC production, as microbial activity may significantly alter nitrosation pathways. By incorporating dietary complexity and microbial interactions, future studies can provide a more accurate assessment of dietary nitrite exposure and associated health risks. It is also essential to consider the changes in the health of the gut microbiome (e.g., healthy versus an IBD individual) which may affect ADME and the overall outcome of exposure.
Future research should also consider the interactions of nitrites, nitrates, and other alternatives on the oral and gut microbiome. The health of the GI tract may influence the interactions of nitrates and nitrites and the formation of nitrosamines, and these effects may be exacerbated by other confounding factors such as cooking temperature and duration.
Despite the extensive studies on the conversion of nitrates and nitrites into harmful NOCs and N-nitrosamines during cooking and food processing, several gaps remain. One gap is the lack of comprehensive data on the effects of different cooking methods and temperatures on the formation of specific nitrosamines across various meat products. While studies have shown that higher cooking temperatures and longer cooking times generally increase nitrosamine formation, the specific mechanisms and interactions between different types of nitrosamines and cooking conditions are not fully understood. Additionally, the impact of various additives, such as antioxidants and starter cultures, on nitrosamine formation needs further exploration. For instance, the varying effects of sodium ascorbate on different nitrosamines suggest complex interactions that require more detailed investigation. Finally, a recurring theme throughout the findings has been the lack of standardised methodologies for the measurement and analysis of key compounds (e.g. NOCs). Although this falls somewhat outside the scope of specific research questions for this review, establishing standardised protocols would be a critical enabler for future studies, ensuring consistency, reliability, and comparability of data across studies and settings.
Future research should investigate the mechanisms by which different cooking methods, temperatures and duration, and additives influence nitrosamine formation. This includes examining how bioactive compounds like antioxidants inhibit nitrosamine formation and their interactions with nitrites during cooking. Additionally, studies should explore the effects of varying cooking intensities on nitrosamine levels in meat products and the potential of natural plant extracts and polyphenols to reduce nitrosamine formation. Understanding the genetic and metabolic pathways of beneficial bacteria, such as Lactiplantibacillus plantarum, in nitrite degradation can lead to innovative strategies for safer food processing. A multidisciplinary approach integrating food science, microbiology, and molecular biology is essential to advance our understanding and mitigation of nitrosamine formation.
There has been extensive testing of alternatives generated from organic sources in an attempt to remove or reduce consumption of inorganic nitrate and nitrite additives in food. However, this research is still in its early stages and needs further development to understand potential risks of the proposed alternatives. For example, there is a need to assess the nitrite degradation kinetics of products developed using both traditional and alternative additives, from processing through storage to consumption, as well as the subsequent formation of nitrosamines. There is also a need for consistency across studies to test a standard set of criteria (e.g. growth or inhibition of specific microorganisms or concentration of specific NOCs) to constitute the evidence of a functional alternative. Additionally, research should explore potential additive or synergistic effects arising from multiple sources of exposure, including both food additives and their alternatives, to better inform comprehensive risk assessments.
There is also a need for research on nitrates and nitrites tailored to the UK population to account for regional dietary patterns, food processing and cooking practices, and exposure levels that may differ from those in existing international studies. Understanding how these compounds interact with the microbiome and contribute to health risks within the specific context of UK consumption can inform more accurate risk assessments and policy decisions.
In addition, there is an opportunity to conduct a more detailed SWOT (strengths, weaknesses, opportunities, and threats) analysis on the alternatives to the nitrates and nitrites as food additives in light of the general population and subsets of the population who may have a higher exposure.
Finally, there is merit in conducting future literature reviews to overcome the limitations of this study. First, a review could be undertaken specifically focussed on data from animal studies which could be translated to human health, as this would extend the findings from the epidemiological and in vitro studies included in this review. Second, another review could explore literature on human studies published in journals with lower impact factor, which were excluded from the current review. This source of data could not be included in this study given the short-term nature of this project, but could further improve our understanding of the safety of nitrates and nitrites as food additives.
5. Conclusion
The review has successfully met the aim of this study in providing an up-to-date synthesis of evidence on the safety of nitrates and nitrites as food additives. Through a structured literature search and the inclusion of 68 robust studies published between 2016 and 2024, the review addressed all key research questions, including those relating to ADME, toxicological risks, processing effects, occurrence in foods, and the viability of potential alternatives. These findings expand the evidence base and identify current knowledge gaps, thereby supporting the FSA’s goal of maintaining an informed and current understanding of nitrate and nitrite safety.
The review has highlighted the benefits associated with the use and study of nitrates and nitrites, illustrating the importance of balancing their useful food safety functions with any increase in potential health risks. In particular, their primary technological function - inhibiting the growth of harmful microorganisms while preserving desirable characteristics such as colour and flavour - remains effective at permitted levels of use. There is currently no clear evidence to suggest significant health risks to the general population from use within these limits, consistent with prior evaluations (e.g. Mortensen et al., 2017b). The literature also identified possible benefits from certain natural alternatives, such as plant-derived compounds with antioxidative or nitrite-scavenging properties, which may reduce the formation of harmful NOCs in foods. However, findings across these studies were mixed and require further validation. Future reviews could look to incorporate animal studies, as this review only included research based on human data.
The review identified notable variability in research methodologies and reporting standards, which complicates interpretation and limits comparability across studies. For example, different researchers applied varied criteria to evaluate the efficacy of alternatives, and some studies reported only residual nitrite levels without detailing the initial amounts added. The absence of standardised methods for measuring and analysing key outcomes, such as specific nitrosamines, contributes to these challenges. Greater consistency in reporting, including the adoption of harmonised units, definitions, and protocols, would enhance the transparency and utility of future research and strengthen the evidence base for regulatory risk assessments.
Several issues of concern and evidence gaps were identified including health risk uncertainties, occasional regulatory exceedances, and biological variability in response. While some epidemiological studies reported associations between higher nitrate or nitrite intake and an increased risk of developing certain cancers, including colorectal, breast, and thyroid cancer, other studies found no significant relationships. These inconsistencies, coupled with modest effect sizes, mean that causal links remain unconfirmed. The review also confirms that higher cooking temperatures, extended cooking times, and the use of nitrite curing salts can increase nitrosamine formation, with mitigations from industry practice and scientific research including measures such as incorporating antioxidants like ascorbic acid, refining cooking processes, and monitoring nitrosamine levels in products. Exposure assessments, particularly from European countries, have indicated that some consumer groups, especially young children with high processed meat intake, may exceed acceptable daily intakes for nitrites. This reinforces the importance of food safety monitoring and compliance with legal limits. Additionally, the review draws attention to the emerging role of the oral and GI microbiome in mediating nitrate and nitrite metabolism. Oral bacteria are key to converting nitrate to nitrite, influencing systemic exposure and the formation of NOCs, while nitrite exposure itself may affect gut microbial composition and activity. These interactions vary between individuals and represent an important area for future research, with in vitro and in vivo studies needed to better understand the implications for health.
The outcomes of this review provide updated evidence to support the FSA and other stakeholders in the risk assessment and regulatory decision-making, particularly in light of evolving international standards. The findings on exposure, health risks, and alternatives may inform future policy and product reformulation efforts. While research on practical alternatives remains ongoing, the review highlights inconsistencies in testing approaches and the need for further validation of their safety and efficacy. The findings also support existing public health messaging, including the importance of a balanced diet rich in vegetables and moderation of processed meat intake, to help maintain nitrate and nitrite exposures within established safe limits. Further research is also needed to clarify how processing methods and ingredient interactions influence nitrosamine formation, particularly under gastric conditions.
Taken together, these findings provide a foundation for future scientific and regulatory work to ensure the continued safety of nitrates and nitrites as food additives in the evolving dietary and policy landscape.
Acknowledgements
We would like to thank our colleagues who made a significant contribution to the project and authored this report, particularly Donna Webley, Emma Sutton, Dr Shraddha Kaur, Steve Hodgson, Sofia Reva and Will Harris at RSM.
We also thank our advisors, Dr Duane Mellor and Professor Gunter Kuhnle, for their valuable direction and guidance at each stage of the project. They provided quality assurance for the literature search strategy, overall methodology and peer-review of the draft and final report to ensure that any information about the safety of nitrates and nitrites as food additives was as accurate and robust as possible.
We would also like to thank Rachel Posaner and Christian Bohm from Knowledge Evidence Services at University of Birmingham for conducting the literature search for this project.
We would also like to thank the Food Standards Agency team for their support and guidance throughout the project, particularly Alba Ureña Rusillo and Claire Potter.
Finally, we would like to express our gratitude to the panel of experts who supported the development of the research questions, provided feedback on our search strategy, shared key sources of information and engaged in productive and reflective discussions on the findings and what they mean for the FSA.
This project was funded under the grant code FS900554.
Appendix A: Search protocol
Search protocol overview
Research aims
The core focus of this research will be to search, gather, review, synthesise the most up-to-date legislation and scientific literature of the four currently authorised nitrates and nitrites food additives and their safety to the consumer: potassium nitrite (E 249), sodium nitrite (E 250), sodium nitrate (E 251) and potassium nitrate (E 252). This will provide an updated evidence base on the absorption, distribution, metabolism and excretion (ADME) and toxicological profile of these compounds and their metabolites and the level of their occurrence in all applicable food categories for human consumption. This information will help to update the knowledge of these food additives and to inform future risk assessments and risk management and wider regulatory policy decisions and guidance. Ultimately, this information will contribute to the FSA’s own mission to ensure food is safe for UK consumers. Table 3 outlines priority research questions for this review.
Clarification of terminology
For this project, nitrates and nitrites refer to the following four compounds: potassium nitrite (E 249), sodium nitrite (E 250), sodium nitrate (E 251) and potassium nitrate (E 252).
The term “food additive” includes consideration of cases when the compounds are added into food sources. This could include where naturally occurring nitrates and nitrites are used directly or indirectly (e.g., concentrated) by the food industry to create, enhance or process food. We will exclude cases where nitrites and nitrates are naturally occurring in food and have not been externally added.
Microbiome includes consideration of both the gut and the mouth microbiome.
Protocol for searching, screening and reviewing the literature
Stage 1. Database searches
We will be reviewing relevant literature from two sources. The first source is academic literature which has been published in scientific journals. The second is grey literature which originates from the UK government and other public agencies government sources (e.g. Canada, Japan, Australia and New Zealand). Rachael Posaner and Christian Bohm (University of Birmingham, Knowledge, and Evidence Services (KES)) will conduct the search for published/academic literature via the University of Birmingham Library Services based on agreed search terms, whilst RSM will conduct the search for grey literature and manage the wider call for evidence. We will use the PRESS checklist[1] to structure our search strategy and fully optimise the time available for the search.
Our advisors Professor Gunter Kuhnle and Dr Duane Mellor will be asked to contribute with any key sources, including those not yet published, available to them given their academic knowledge and network. In addition, RSM will also issue a call for evidence and ask the FSA expert panel and our advisors to disseminate this call for evidence.
We propose the following search criteria and databases. For the majority of our search terms, we will search across the title, abstract, author keywords, and relevant MeSH headings to capture a broad set of relevant articles. However, for certain broader or more generic terms, we will restrict the search to just the title and author keywords (as well as relevant MeSH terms, when applicable). These terms will include “nitrate”, “nitrite”, “toxicology”, “absorption”, “occurrence”, “concentration”, “exposure”, “alternative”, and related terms, with MeSH headings used for terms such as “nitrate”, “nitrite”, and “toxicology”. This approach will help minimise irrelevant results by focusing on the most relevant fields to ensure specificity. Parameters will be refined depending on the availability of relevant sources. The literature search will commence from January 2016 to align with the period following EFSA’s 2017 re-evaluation. While this may exclude some earlier foundational studies, it is deemed appropriate to ensure the review reflects the current context and most relevant evidence. Table 4 outlines search terms and inclusion criteria for this review.
Stage 2. Screening of titles and abstracts
Using our various sources of literature, we will review a longlist of a maximum 600 titles of published and unpublished studies, articles and reports (‘grey literature’) pertaining to the research questions on the safety of nitrates and nitrites as specified above. Table 5 below sets out the first level inclusion/ exclusion criteria which we will apply to each title. We anticipate excluding 25% to 50% of titles at this point either because they are not of central relevance to the four authorised food additives, or they are duplicate studies in our sample.
We will then review around 300 abstracts and executive summaries at the second stage of screening, having already excluded irrelevant titles and duplicates. The second level criteria are listed in Table 6 below and relate to the detailed research questions. These may need to be refined depending on the number of studies retrieved. Relevance of the evidence for each research theme will be assessed on a 3-point scale (from 1=low to 3=high), considering to what extent does this text help to provide an evidence-based answer to the research question/s that come under this theme. Abstracts which receive a 1 across all research questions will be discarded and the remaining abstracts will form the shortlist of relevant literature for further screening and quality assessment.
Stage 3. Quality assessment of full texts
Quality appraisals will be completed concurrently with the extraction process. We will ensure that our work meets quality ratings according to GRADE[2] and AMSTAR II[3] (for academic literature). For grey literature, this will only be screened based on its relevancy to the research questions and selected quality criteria (e.g. credibility of source; risk of bias). During quality appraisal, particular attention will be paid to how funding sources (e.g. industry vs. public health) may influence study outcomes. After removing irrelevant abstracts, we expect to shortlist about 60 full texts. They will be examined and screened to identify the final list of the most relevant, informative and useful studies.
Relevance of the evidence for each research theme will be assessed on a 3-point scale, (from 1=low to 3=high), considering to what extent does this text help to provide an evidence-based answer to the research question. Robustness of the evidence will be measured on a 3-point scale (from 1=low to 3=high). Table 7 outlines the rating system.
Throughout the search process, a log will be kept on a spreadsheet which will eventually be developed into the full literature review log in the next stage of the search protocol.
Stage 4. Data extraction
We will complete a data extraction for a maximum of 60 papers or reports, and extract information from the review literature into a spreadsheet which can be filtered for each research theme. The suggested headings for the spreadsheet are below. If after reviewing all full texts significant gaps in the literature remain, it may be required that we conduct a purposive second round search to gather new information. To keep our approach structured, we will follow the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting items[4] to allow for a transparent, encompassing and comparable collection of article records included in our final review list. The following headings will be incorporated during data extraction:
-
Document Title
-
Author(s)
-
Date of Publication
-
Organisation/owner
-
Country
-
Type of study
-
Quality appraisal
-
Source of funding
-
Author conflict of interest
-
Selection/attrition bias
-
Sample size
-
Confounding bias
-
Reporting bias
-
Bias (different for each study type)
-
-
Relevancy mapping
-
RQ1
-
RQ2
-
RQ3
-
RQ4
-
RQ5
-
-
Inclusion
In addition to the above headings, the extraction headings included:
-
RQ1 New Evidence
-
Absorption
-
Distribution
-
Metabolism
-
Excretion
-
Influence of mouth microbiome
-
Influence of gut microbiome
-
-
RQ2 New Evidence
-
Related to cancer risk
-
Related to diabetes risk
-
Related to risk of other diseases
-
Other effects on the body e.g. migraines
-
-
RQ3 New evidence
-
Cooking
-
Food processing
-
Country-specific cooking practices affecting conversion of nitrates and nitrites into nitrosamines
-
-
RQ4 New evidence
-
Presence in authorised food categories
-
Presence in unauthorised food categories
-
-
RQ5 New evidence
-
Complete replacement of nitrates/nitrites
-
Partial replacement of nitrates/nitrites
-
Synthetic additives
-
Natural additives e.g. plant extracts
-
-
Findings from Previous Research / Context (for each RQ)
-
Methodological Uncertainty
-
Contradictory / Conflicting Findings
-
Extraction will take place using a Systematic Review Data Repository extraction tool. Findings across the evidence base will be synthesised following ESRC guidance on conducting narrative synthesis[5]. If conflicting evidence arises as part of the review, we will highlight where conclusions diverge presenting both viewpoints on the evidence considering the weight of evidence and highlighting areas where further research is needed. For example, outlining which studies found evidence of potential health risks (e.g. cancer or cardiovascular effects) and which studies did not. Additionally, we will report any biases identified through quality appraisal and will discuss the limitations that arise from conflicting evidence or methodological shortcomings.
Synthesis will be grouped by research question and will provide single-sentence evidence statements for each. Within this synthesis, information on the volume and quality of evidence per research question will be included, highlighting gaps where they exist.
PRESS 2015: checklist for search strategies | Karolinska Institutet University Library
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. Available here.
Popay et al. (2006) Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Institute for Health Research, Lancaster University