1. Executive Summary
The fundamental mission of the FSA is food you can trust. Edible oils comprise a commodity for which some of the highest numbers of adulteration incidents are recorded internationally. Oils can be diluted with, or substituted for, undeclared oil for economic gain. This is a concern for consumer safety, food authenticity and consumer trust in the food supply chain.
The scope of this project was to identify and review current and emerging methods to detect adulteration in edible oils, to analyse gaps in capability and knowledge and to conclude with recommendations to support edible oil authenticity in the future. This involved a critical and unbiased literature review, stakeholder engagement, consultation of proficiency testing data and consultation of HorizonScan™ data to understand future risks. There is greater focus on the types of adulteration which are important to UK consumers and to the UK economy. There is therefore more emphasis on detecting adulteration due to substituting or diluting edible oils with oils of a different botanical origin or with non-food grade oils rather than detecting issues relating to protected designated origin status, geographical origin or level of refining.
The number of global incidents of oil authenticity issues is increasing. In addition to misrepresentation of oil botanical origin, this includes an increasing trend of incidents involving presence of mineral oil, re-used/gutter oils and Sudan dyes in edible oil. These issues pose a serious health and safety concern.
The literature review highlights that approaches to determine authenticity range from methods with a rapid, point-of-contact ambition, often based on spectroscopic technologies, to methods aimed at confirmation, including the profiling of fatty acids, sterols and triacylglycerols. The publications identified make use of methods that are in their relative infancy, often not having been tested on a wide range of oils taken from a collection of suppliers and sources, or accounting for seasonal variation and not having undergone collaborative trials. There is a concerning lack of uptake in proficiency testing trials and a lack of accuracy in the methods used.
An issue that became increasingly apparent was the perceived high levels of sophistication of fraud in edible oil supply. This was widely recognized among the project’s stakeholders, and has led many businesses to develop testing methods independently, often in isolation. Unfortunately, this siloed approach is not only delaying the development of effective fraud detection techniques but also hindering the establishment of industry-wide standardisation.
This report makes recommendations to support consumers, the edible oil industry and the associated UK economy into the future. There is a lack of standardisation and also regulation regarding edible oil and these areas must be addressed. Investment is required to support widespread testing, to develop point-of-use methods and to develop confirmatory methods. The validation of these methods is key to understand measurement uncertainty. Spectroscopy methods such as Fourier Transform Infrared and Raman showed potential for future investigation as rapid, low-cost point-of-use methods. Analysis of triacylglycerols demonstrated strong potential to comprise a confirmatory method which would be accessible to Official Laboratories to support enforcement. It is also required that authentic certified reference materials are prepared to support quality control in testing. This should provide confidence in data and encourage future levels of uptake in proficiency testing to support testing activities.
2. Abbreviations
δ18O: Measure of the deviation in the ratio between stable isotopes of oxygen-18 and Oxygen-16
δ2H: Measure of the deviation in the ratio between stable isotopes of Hydrogen-2 and Hydrogen-1
µg/g: microgram per gram
AOAC: Association of Official Agricultural Chemists, International
AOCS: American Oil Chemists’ Society
APCI-IT MS3: Atmospheric-Pressure Chemical Ionisation Source Ion Trap Mass Spectrometry
ASAP: Atmospheric pressure Solids Analysis Probe
ATR: Attenuated Total Reflectance
CAD: Charged Aerosol Detector
CDA: Canonical Discriminant Analysis
CoE: Food Authenticity Network’s Centre of Expertise
CRM: Certified Reference Material
ECN: Equivalent Carbon Number
DAG: Diacylglycerol
DART: Direct Analysis in Real Time
Defra: Department for Environment, Food and Rural Affairs
DNA: Deoxyribonucleic acid
dsDNA: Double stranded DNA
EI: Electron ionisation
EPR: Electron Paramagnetic Resonance spectroscopy
ESI-HRMS/MS: Electrospray High Resolution Tandem Mass Spectrometry
ESR: Electron Spin Resonance spectroscopy
EVOO: Extra Virgin Olive Oil
FA: Fatty Acid
FAEE: Fatty Acid Ethyl Ester
FAME: Fatty Acid Methyl Ester
FFA: Free Fatty Acid
FID: Flame Ionisation Detection
FIR: Far-Infrared (Spectroscopy)
FSA: Food Standards Agency
FT: Fourier Transform
FTIR: Fourier Transform Infrared spectroscopy
GC-MS: Gas Chromatography Mass Spectrometry
GC-FID-MS: Gas Chromatography Flame Ionisation Detection Mass Spectrometry
GC x GC: Two-dimensional Gas Chromatography
GO: Geographical origin
HCA: Hierarchical Cluster Analysis classification algorithm
HPLC: High Performance Liquid Chromatography
HRM: High Resolution Melting
IC: Ion Current
INEPT: Insensitive Nuclei Enhanced by Polarisation Transfer
IR: Infrared (Spectroscopy)
IRMS: Isotope Ratio Mass Spectrometry
IOC/COI: International Olive Council
ISO: International Organisation for Standardisation
LDA: Linear Discriminant Analysis
LC-CAD: Liquid Chromatography with Charged Aerosol Detector
LC-GC-FID: In-line Liquid Chromatography Gas Chromatography Flame Ionisation Detection
LC-MS: Liquid Chromatography Mass Spectrometry
LDA: Linear Discriminant Analysis
LOO: Lampante Olive Oil
LOQ: Limit of Quantification
MAG: Monoacylglycerol
MALDI-ToF: Matrix-Assisted Laser Desorption/Ionisation-Time of Flight
MDGC: Multidimensional gas chromatography
MIR: Mid Infrared Spectroscopy
MOSH: Mineral Oil Saturated Hydrocarbons
MOAH: Mineral Oil Aromatic Hydrocarbons
MUFAs : Monounsaturated Fatty Acids
m/z: mass/charge ratio
NIR: Near Infrared (Spectroscopy)
NMR: Nuclear Magnetic Resonance
OA: Oleic acid
OL: Official (Control) Laboratory
OO: Olive oil
OOO: Organic Olive Oil
OPLS-DA: Orthogonal Partial Least Squares Discriminant Analysis
PA: Public Analyst
PCA: Principal Component Analysis
PCR: Polymerase Chain Reaction
PDO: Protected Designated Origin
PGI: Protected Geographical Indication
PLSR: Partial Least-Squares Regression analysis
QC: Quality Control
PDO: Protected Designated Origin
pg: picogram
PGI: Protected Geographical Indication
PT: Proficiency Test
PUFAs: Polyunsaturated acids
PV: Peroxide Value
qPCR: Real-time PCR
R&D: Research and Development
RF: Random Forest classification algorithm
RM: Reference Material
R-SVM: Recursive Support Vector Machine discriminant model
SIRA: Stable Isotope Ratio Analysis
sn: Stereospecifically numbered carbon atom
SNP: Single nucleotide polymorphism
SOP: Standard Operating Procedure
SORS: Spatially Offset Raman Spectroscopy
SPE: Solid Phase Extraction
SPME: Solid Phase Multi-Extraction
SSR: Short tandem repeat
TAG: Triacylglycerol
THz: Terahertz
UHPLC : Ultra-High Pressure Liquid Chromatography
UV: Ultraviolet
VOCs: Volatile Compounds
VOO: Virgin Olive oil
3. Introduction
The fundamental mission of the FSA is food you can trust. Suitable analytical methods are required to ensure that food is what it says it is as declared on the food label and to manage risk around food authenticity. FSA aims to ensure that Public Analyst Official Laboratories are adequately prepared with the most effective technology and methods for FSA surveillance and food law enforcement testing.
Edible oils are one of the food commodities for which the highest numbers of adulteration incidents are reported. The combined Europol and Interpol operation against food fraud (December 2020 to June 2021), known as OPSON X, highlighted cooking oils as one of the most adulterated foods in Europe. Common fraud issues include mislabelling, adulteration of ingredients and misrepresenting product geographical origins when protected. Quality parameters vary, including nutritional levels, purity, absence or presence of contaminants and stability to heating or oxidation during storage. Quality is impacted by a number of factors including the level of refining method used to prepare the oil, processes used to clean the oil, the botanical origin (olive, rapeseed, palm, etc.), cultivar and climate factors during the ripening period. Cold-pressed oils for example, are obtained by mechanical procedures only without altering the oil, for example using expelling or pressing without application of heat and may then be purified by water washing, settling, filtering and centrifugation only (standard for named vegetable oils, Codex standard 210-1999, adopted (Codex Alimentarius, 2024)). Cold-pressed oils often carry a high economic value. Refined oils on the other hand may suffer loss of nutritional content and tend to carry a lower economic value.
Oils of certain botanical origin, particularly olive oils, can command a higher value at market than many other oils due to related nutritional and reported health benefits. This makes olive oils particularly vulnerable to adulteration events such as dilution and substitution or mislabelling. The risks to the authenticity of edible oils are also further impacted by the effects of weather events on harvest quality and volume and the changing political environment on global supply chains. Both of these issues can impact the availability of oil and therefore its market value. A cost-of-living crisis can also alter demand, and in turn value, of certain edible oils and impact their vulnerability to fraud.
Adulteration of oils has the following main impacts:
- A health issue due to unexpected content within an oil, which at worst can impact safety (relating to the presence of undeclared allergens, dangerous chemical compounds or non-food-grade oils) and at best can impact health relating to the nutritional composition of an adulterated oil.
- Economic impact on suppliers due to unfair competition by adulterated products.
- Misleading of consumers and loss of consumer confidence in the food supply chain.
- Economic impact on consumers who do not receive the product that they have paid for and may be disappointed with the product quality.
- Legal issues due to breaking of the regulations relating to edible oil supply.
- Ethical impacts, including legal aspects and also adulteration by ethically unsound oils such as palm oil from unsustainable sources.
Regarding regulation, edible oils are covered in UK by the Food Safety Act of 1990. The Olive Oil (Marketing Standards) Regulations 2014 (Statutory Instruments 2014/195) is the UK regulation which provides the legislative basis for the compliance regime for implementing in GB. The assimilated EU regulations 29/2012 Marketing Standards for Olive Oil and 2568/91 Characteristics of olive oil and olive residue oil and the relevant methods of analysis also apply. In Northern Ireland, Commission Delegated Regulation (EU) 2022/2104 marketing standards for olive oil, and Commission Implementing Regulation (EU) 2022/2105 laying down rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil apply. A Codex Alimentarius Commission standard is also in place for edible oils (standard for named vegetable oils, Codex standard 210-1999 (Codex Alimentarius Commission, 2024)). While regulation is mandatory, Codex standards are not.
This project builds on previous Defra-funded projects:
-
FA0117, Evaluation of methodology to verify oil species in mixtures of oil 2012-2013 and associated SOP (reference FA0158), which used a combination of Fourier transform-infrared (FT-IR) and Raman spectroscopy as screening methods followed by FAMEs as a confirmatory method to verify edible oils species, plus development and validation of the proposed methodology to verify vegetable oils species in mixtures of oils and
-
Q01095, Validation of a DNA-based method for the determination of hazelnut oil in olive oil, 2008.
The ability to test oils for their authenticity is critical to protecting consumers and the food supply chain alike. This project has been funded by the FSA to provide an independent review of the current status of edible oil authenticity testing and to identify methods which will help to improve capability in Public Analyst (PA) Official Laboratories (OL).
4. Key Project Deliverables
The project comprises:
-
Initial expert consultations with two specialists, one involved in edible oil supply and one in analytical techniques.
-
An international critical and unbiased review of published literature to identify current and emerging analytical methods suitable for the analysis of oils of particular interest, capturing data on analytical method performance, knowledge gaps, and challenges (sensitivity, throughput, cost-effectiveness, etc.)
-
Access to HorizonScan™ data to inform regarding international edible oil authenticity events which have been reported by international governments by official means over the last 10 years. These data can be used to help to predict future vulnerabilities to support the FSA in future-proofing the UK edible oil industry.
-
Access to Fapas® Proficiency Testing (PT) oil authenticity data to understand proficiency testing uptake and accuracy of testing.
-
Detail databases and Quality Control standards used to underpin testing.
-
Extensive stakeholder engagement with enforcement, testing laboratories, industry suppliers and research laboratories to understand the current situation:
a. Capturing current analytical methods and available technology/ equipment.
b. Identifying knowledge gaps and challenges.
-
Evidence-based recommendations:
a. To further protect the supply chain.
b. For improving Official Laboratory capability for oil authenticity testing.
c. For further research and development in this area.
5. Consultations with specialists in edible oil supply and analytical techniques
5.1. Consultation 1. Adulteration prevention consultation with food industry R&D specialist
A lipid specialist with over 30 years of experience in analytics of foods was consulted regarding recommended approaches within supply chain to protect the authenticity of edible oils. The outcomes of the consultation meeting are outlined below.
5.1.1. Current status of authenticity in edible oil supply
Given consideration that fraudsters in food supply are becoming highly sophisticated, oil suitability testing approaches are available to detect authenticity issues in the supply chain. These approaches need to be constantly adapted to address new scenarios, some being purely hypothetical but often realistic under certain circumstances. Proactivity is essential in this domain. The importance of food supply companies keeping one step ahead of fraudsters to protect oil authenticity was stressed during the consultation, to protect manufactured food products, avoid consumer and authority complaints and ensure consumer safety.
The consultant highlighted that edible oil adulteration is increasing in frequency and is not always associated to the financial aspects (i.e. economically motivated adulteration or EMA) often observed in recent decades, but driven by other factors (i.e. consequence of war, shortage of certain edible oils, climate change, etc). The likelihood of certain forms of authenticity frauds used in the past should be reviewed regularly and updated since new scenarios could be significantly different to those which have been reported historically.
5.1.2. Recent cases of adulteration
Please note that some of the information discussed in this consultation is hypothetical and not guaranteed as final conclusions were never given officially.
Ukraine is the leading producer of sunflower oil globally and, following the inception of war in 2022, its availability sharply declined, impacting oil supply chains and driving up global edible oil costs. The food industry must adapt its production to fluctuating oil availability in the markets, which opens up new possibilities for oil adulteration and substitution scenarios that were previously never considered or imagined, affecting even olive oil.
In 2008, mineral oil was found in massive concentration in Ukrainian sunflower oil shipped to France and then distributed across Europe. Mineral oil was detected only after having already manufactured food products using this oil. This incident highlighted the risks occurring when the quality and/or composition of incoming materials is not tested upon receipt, or when the methods used for testing incoming oil are not able to detect such contamination. At that time, analytical methods already existed to detect Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH) at levels of 50 mg/kg, for example by in-line Liquid Chromatography Gas Chromatography Flame Ionisation Detection (LC-GC-FID) but the method was not implemented in many labs. Such incidents have initiated huge and continuous efforts during the last 20 years to set up and update the analytical methods to quantify increasingly lower levels of mineral oil, but also mitigate the contamination with oil suppliers and adapt the regulation at the same time. The limit at the time of consultation (Autumn 2024) for MOSH is 2 mg/kg and that for MOAH is 0.5 mg/kg.
A significant safety concern has emerged in China regarding the reintroduction of used frying oil commonly referred to as gutter oil, into the supply chain. This practice raises the possibility of mixing or substituting gutter oil with fresh oil for adulteration purposes. According to videos shared by various media outlets, the appearance of this oil resembles paint and appears unsuitable for reintroduction into food products. However, after undergoing filtration and the removal of degraded components, followed by a re-refining process, the oil can become clear and transparent, similar to other vegetable oils. This oil contains burnt residues from frying products that may include carcinogenic substances. It is heavily degraded and oxidized, exhibiting high polar content and degraded triglycerides. As a consequence, traditional analytical tools could be inefficient to detect the presence of reused oil such as refined gutter oil, if markers have been eliminated from the oil using a specific process. Sourcing gutter oils from reputable suppliers for industrial use is practically impossible, and it seems more likely that they are consumed in smaller markets, such as mobile kitchens.
5.1.3. Approach in sourcing edible oils and fats
As part of the risk assessment to protect the integrity of the oils sourced, a ‘requirement document’ (detailing all requirements and compositional needs of the oil being purchased) should be prepared by the sourcing party. This document should provide detailed information about the desired characteristics of the purchase materials. For oil and fat, this includes the origin (i.e. botanical origin, cultivar country), the composition (e.g. fatty acid content), the quality (i.e. peroxide value (PV), free fatty acids (FFA)) and the physical properties (i.e. Solid Fat Content, Melting point if the oil or fat is solid at room temperature), but also limits for a long list of contaminants which are strictly regulated, for example Maximum Residual Levels (MRL) of contaminants such pesticides, Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH) or process contaminants such as 3-monochloropropane-1,2-diol (3-MCPD) and glycidyl esters. Depending on the type of oil, other parameters can be introduced. Fixed limits (i.e. minimum and maximum values) must correspond to the desired material but should also include the natural variabilities. A well-designed requirement document can prevent food fraud (this document can be considered as the 1st layer of adulteration prevention). Any adulterated oils would therefore need (in principle) to mimic the exact composition requirements of the ‘requirement document’ which would be challenging for fraudsters to achieve. However, software (based on an oil composition database) has existed for years and can be used to design an oil mix composition based on the fatty acid composition of each oil. Then sourced oil and fat need to be analysed by oil suppliers and a certificate of analyses should be delivered. It is almost impossible to analyse all parameters in each batch of oil and fat, but for this reason, release parameters (mandatory) for the most important parameters need to be fixed.
5.1.4. Additional requirements
This consultant recommended that oil purchasers must routinely check the certificates which accompany the oils they source since the certificate may in fact fraudulently or accidentally relate to a different batch of oil compared to the batch received. Companies can invest in analytical testing to interrogate the information on batch certificates to protect the company’s reputation. As part of this brand protection, companies could support the harmonisation of methods and also encourage their suppliers to participate in proficiency testing schemes to increase supplier confidence in the authenticity of the oils that they procure. To do this, financial and training support could be provided to suppliers to support oil authenticity.
5.1.5. Edible oil and fat testing methods
Analytical methods for olive oil, adopted by the International Olive Council (IOC/COI), are often the most advanced for authenticity aspects compared to other botanical types of oil. More development is justified by the relative high costs of olive oils compared to other edible oils, but also due to the fact that olive oil is classified in 8 different categories, and this has a huge impact on the risks of non-compliance or frauds. It is not a surprise that olive oil frauds began some centuries ago, but now such frauds are becoming highly sophisticated. In addition, olive oil is impacted by the variations of the composition of over 100 cultivars used in a single varietal oil or in a blend which can originate from different countries but is also impacted by weather conditions and climatic changes. Also, the risk of olive oil fraud is increased greatly due to the availably of other vegetable oils having a very similar composition to olive oil. High oleic oils are one such example, including high oleic sunflower oil which has been used to substitute olive oil for decades. Other high oleic oils are now coming to market. Avocado oil is such an example.
The methods developed for olive oil composition and quality are often similar to those used for other vegetable oils and fats registered with International Organisation for Standardisation (ISO), American Oil Chemists’ Society (AOCS), Association of Official Agricultural Chemists (AOAC International). This is of no surprise as most of these methods originated from International Union of Pure and Applied Chemistry (IUPAC). However, these methods are intended to analyse the oil composition (fatty acid, sterol), quality (PV, FFA). They do not provide guidance for adulteration detection as such. This requires the help of experts with access to a robust oil and fat database.
A large range of analytical methods exists using different instruments (simple to more sophisticated) and can be used for authenticity aspects. They can be used to detect adulteration or contamination and could be suitable to address low limits of quantification. The variation of oil and fat composition is not influenced by the same factors observed for olive oil, because other edible oils generally originate from one unique cultivar, and other oils are refined.
A scenario which can lead to increased complexity in authenticity testing is the possibility of fractionating the oil (for example, palm oil) to obtain the desired physical properties (sometimes unique properties).
The existing standards to detect foreign fat (adulteration) are:
ISO 23275-1 Animal and vegetable fats and oils - Cocoa butter equivalents in cocoa butter and plain chocolate - Part 1: determination of the presence of cocoa butter equivalents
ISO 23275-2 Animal and vegetable fats and oils — Cocoa butter equivalents in cocoa butter and plain chocolate — Part 2: Quantification of cocoa butter equivalents
ISO 17678 I IDF 202 Milk and milk products — Determination of milk fat purity by gas chromatographic analysis of triglycerides
ISO methods such as fatty acid profiling by chromatography are generally the primary methods which are used to detect adulteration. Then diagnostic testing can be completed by other methods such as triglycerides profile, sterols, tocopherols/tocotrienols or by the analysis of unsaponifiable compounds.
Other routes can be used for olive oil testing. Specific methods have been developed by the International Olive Oil Council (IOC) for this purpose such as:
-
GC-FID of fatty acid methyl esters (FAMEs) in olive oil.
-
Determination of waxes and fatty acid methyl and ethyl esters in olive oils to distinguish between olive oil and olive pomace and detect fraudulent mixtures of extra virgin olive oils.
-
Triacylglycerol (TAG) composition to determine olive oil authenticity for detection of organic olive oil (OOO).
-
Fatty acid profiling.
-
Solid phase multi-extraction (SPME) coupled with GC-FID mass spectrometry GC-FID/MS to analyse the volatile profile of olive oil to verify correlation with sensory panels.
5.1.6. Standardisation of methods
Standardisation of methods is a very important activity to provide suitable methods to characterise oil composition to contribute to authenticity testing of oils and fats. Analytical methods aiming at the analysis of the fatty acids in the stereospecifically numbered carbon atom 2 (sn-2) position of the TAG molecule is one example (possible on a sophisticated LC-MS-MS instrument, but sn-2 can be also analysed by GC-FID (ISO 6800, 6062).
Regulatory laboratories are included in the standardisation process to align manufacturers with enforcement authorities and international method harmonisation is key to the protection of oil integrity. It is also important to have Codex Alimentarius endorsement of methods.
5.1.7. Availability of certified reference materials
The consultant highlighted the need to have robust reference methods to detect adulteration. However, the need to have Certified Reference Materials (CRMs) in this area for method implementation and monitoring is essential. The preparation of CRMs requires much time and testing expertise and future financial investment to support CRM preparation for vegetable oils from a range of botanical species. This requires the engagement of experienced participants who are also motivated to be involved in this very important task to protect oil authenticity.
5.1.8. Emerging methods
Spectroscopic methods (i.e. NIR, NMR, Raman) are often cited as the most promising rapid and environmentally-friendly methods to address oil and fat authenticity, using fingerprinting approaches and chemometrics. Even if the suitability of these technologies for oil and fat composition and quality has already been successfully tested, solid calibration models and continuous maintenance are required. Limit of Quantification (LOQ) is sometimes an issue due to interfering compounds. We can understand the reasons for the increase of the use of these technologies since they can replace (as an initial screen approach of oils and fats testing) several analytical methods using chemicals and solvents which are time consuming and less safe, so they can also contribute directly to cost saving. However, it is important that the competencies needed for more complex chemical methods are maintained because the results provided by these methods are used for the calibration of spectroscopic instruments and also chemical methods are necessary for the second (confirmatory) level of testing of oils and fats, if suspect data have been detected by the spectroscopic method (1st level of testing). Chromatographic methods including mass spectrometry tend to have a lower LOD than spectroscopy methods and are used to detect oil adulteration, or even non-intentional cross-contamination, but using different markers. Ideally the revision of the fixed limit for markers in each oil and fat would offer an improvement in testing to help to prevent the fraud or make it less easy for the fraudsters. For instance, Codex has introduced a limit for the fatty acid composition of certain oils such as avocado oil (following issues which arose with avocado oil authenticity). One of markers used for avocado oil is now palmitoleic fatty acid (C16:1 n-7) where the minimal content has been fixed at ≥4%. Should a lower level be found in avocado oil, this could be linked to adulteration with another oil.
As mentioned, the benefits of analysing sterols and tocopherols/tocotrienols to complement authenticity diagnostics could be interesting, but it must be considered that oil refining reduces the concentration of these compounds in the oils and fats and that reliable results are necessary to fine-tune these diagnostic tests regarding adulteration.
5.1.9. Emerging issues
The consultant advocated for using regular horizon scanning for changes in the supply chain, for example linked to altered availability due to climate events, geopolitical events etc. to predict, plan and test for, new risks to edible oils.
5.1.10. Recommendations for the future
Consultant 1 recommended that future development and harmonisation of methods is essential, including the support for infrastructure and expertise necessary for the preparation of CRMs intended to implement and to monitor analytical methods, but also to check the performance of testing labs. Sourcing pure and authentic material to make CRM for authentication can be a very difficult task, especially for refined vegetable oils and fats because they are available in high volume, where the overall content may have originated from different origins, periods of production and suppliers are often not transparent (not willing to be transparent) and cross-contamination can also occur. Support is needed to source authentic oil samples around which to build the datasets which underpin the testing methods. This requires trained and trusted personnel attending seed collection points or plantations to assure that authentic oils can be independently prepared. This kind of approach seems more feasible with small oil producers (since they can monitor the whole process, from seed production to oil refining). Sourcing authentic olive oil can be relatively straightforward in that an olive oil producer can be visited to source the material and small olive oil press companies exist which could be used to prepare an authentic oil of assured origin and cultivar.
The consultant advises that there is a need for companies to complete as much due diligence on oil suppliers as possible to reduce risks of fraud. Non-Government Organisations are becoming increasingly active in consumer protection, to identify issues in food (i.e. contaminants, authenticity and/or origin). It is also critical to ensure that fraudsters do not have access to knowledge regarding methods under development, but this is impossible to guarantee. The consultant emphasised that suppliers cannot rely on food certificates and the paper trail surrounding oil supply. Ultimately companies currently need to take responsibility to ensure they are in a position to protect their own reputation and to protect their brands so that products are prepared in accordance with requirements to ensure they are safe for consumers. Companies must organise independent testing to interrogate the paper trail and to protect reputation. This is a continuous process and testing facilities must keep up to date with known issues and predicted risks occurring in food supply to keep a step ahead of fraudsters and protect their brands.
Finally, this consultant from the industry highlighted the value to the integrity of the food supply industry of future proficiency testing rounds relating to oil adulteration scenarios (i.e. foreign oil added to pure oil) with the intention to verify testing labs’ capabilities in detection of adulteration. Analytical capability must be always a step ahead of fraudsters and not the reverse.
5.2. Consultation 2. Research and development in edible oils: Expertise, experience and vision for rapid and cheap screening of edible oils
Consultant 2, Dr Jackie Mosely, is a specialist in mass spectrometry with an interest in lipidomics, and recent research has focussed on analysis of edible oils to investigate potential markers of origin. The deployment of rapid testing methods which could be used at point-of-use would support the food supply industry and would potentially drive economic growth within food supply. Dr Mosely’s long-term aim is to develop rapid, low cost and portable methods to interrogate the differences to determine reliable markers or ‘signatures’ of markers relating to the type or species of edible oils. Fulfilment of this aim often necessitates initial discovery phase work using high resolution mass spectrometry technologies which produce data less quickly and require a high initial investment followed by transfer of the method to rapid, lower-cost and ideally portable instruments.
Much of Dr Mosely’s research has focussed on analysis of the triacylglycerols (TAGs) which are the main components of the fatty acids within seed oils. TAGs are comprised of three fatty acids attached to a glycerol molecule. Attachment of the component fatty acids can occur at various positions, which in turn determines the physical properties of triacylglycerols. The most common fatty acids contain 12–22 carbon atoms and exist in saturated, monounsaturated, and polyunsaturated forms. The TAG profile of an oil varies with botanical origin. The approach by other researchers is to study TAGs alone. However, Dr Mosely considers that chemical degradation, and sometimes instrument induced fragmentation, may compromise the TAG signature. Therefore, Dr Mosely is studying the relative abundance of diacylglycerols (DAGs), and may also consider monoacylglycerols (MAGs) alongside analysis of these TAGs, as a potentially more informative or more robust analytical approach. Her work is also expanding to investigate diglycerides (or diacylglycerols, DAGs), with DAGs being expected to provide more data to fully characterise oils to support the differentiation of the species origin of oils. Recent major advances in LC-MS have allowed this characterisation of TAGs in oils, with the benefit of chromatographic separation to capture minor components, which has an added benefit over other techniques used in the past such as NMR, MALDI-MS, Raman spectroscopy, and mid-infrared spectroscopy. This chromatographic separation provides a greater sensitivity to detect adulteration, and may provide more accurate results. Recent research in Dr Mosely’s team for example concentrated on investigating adulterated oils. Mixtures of botanical origin could be distinguished from pure oils using LC-MS with chemometric tools (PCA and HCA), even in mixtures of oils with similar compositions of the major TAGs. Oils could also be differentiated as to whether they had undergone long-term storage post-production due to changes in the TAG profile of certain oil types (Alfifi, 2023).
Dr Mosely’s approach has been to involve high resolution mass spectrometers for a robust measurement of the maximum number of components. Patterns in the data between different oil types can then be investigated. As many samples of each oil type as possible are included in the dataset. Once the discovery studies are complete, the aim is to transfer the method(s) onto more rapid (e.g. 5-minute test) and lower cost instruments. This approach aims to simplify the whole analytical process, reduce financial and time requirements which would support high volume testing. Ideally future instruments for initial sample screening would be portable, requiring only minimal sample preparation and could be used reliably in situ during port inspection and in factories or warehouses by trained non-experts. Following successful development of rapid screening methods to be used during ‘in the field’ testing inspection, the composition/authenticity of samples which have provided suspect data could then be further interrogated by confirmatory methods.
5.2.1. Preparation of reference datasets
A key consideration during the method development stage is the reproducibility of oil profiles, for example, of oils of the same type/species but originating from different geographical origins or by different modes of refining. Much consideration is required regarding data processing and the effect of chemometrics, artificial intelligence and machine learning.
When developing suitable methods, Dr Mosely highlighted the vital necessity to prepare a robust reference library. The outputs of the testing method developed are only as accurate as the reference dataset from which the method is built. Such a dataset would ideally be composed of many authentic samples of diverse origins (diverse geographical origins, preparation/manufacturing origins, length of time in storage) and mixtures (for example, comprising a wide range of samples comprising 1-99% of a known oil type mixed with another known oil type) prepared from these authentic samples to build a comprehensive reference database. The authenticity of these types of samples cannot be presumed from the product labelling alone, for example for retail samples and therefore it is unsuitable to base a reference database on retail samples. Instead, much extra effort, often involving trusted inspectors sourcing authentic oils at point of production within the supply chain, is required to gain as much confidence as possible regarding the authenticity of the products which form the basis of a robust reference database. It is anticipated that it will be exemplified in the literature review for this project that authors have relied on retail samples to prepare their datasets and this is a limitation of current testing methods. There is a requirement for the provision of authentic and fully traceable oil samples to support testing methods.
Samples entering the reference dataset should be analysed in triplicate and by more than one analyst, and ideally at a later stage in method development in more than one laboratory, to understand the precision of the data and its transferability to other laboratories.
Before the data from a sample can enter a reference dataset, it would be of benefit to identify a range of standardised industry tests which could be performed on the sample to verify that the oil is actually an oil and to check that there is not too much water present, possibly due to poor storage, especially if the oil is suspected of undergoing long-term storage. There are also considerations for whether there has been a head space of air in the storage vessel which may have caused oxidation of the oil components, whether the oil has been stored in glass or plastic and whether this may affect the composition, perhaps due to presence of plasticisers. Dr Mosely highlighted that, when performing the literature review, it will be interesting to determine whether researchers have detailed how they homogenise oils prior to testing in order to gain a representative food sub-sample for testing as this could compromise data in the datasets described in the literature.
5.2.2. Considerations relating to quantitation, dynamic range and limit of detection.
The differentiation of edible oils is a challenge. It is anticipated that the dynamic range of methods will be determined to some extent by the technologies used. Considerations for Dr Mosely’s research have included reflection over dynamic range, recognising that the required limit of detection (LOD) may differ depending on the oil being identified. The LOD will be required to be very low for oils which pose a safety risk such as mineral oils and gutter (recycled) oils. Any adulteration of edible oils with mineral oil for example poses a serious health and safety risk to consumers due to the toxicity of mineral oil. Mineral oil is an easy oil to detect due to it having a different carbon chain length to edible oils. Other adulteration scenarios pose less of a severe health risk. Dilution of rapeseed oil with another vegetable oil, for example, can impact the nutritional quality of the final oil but does not pose an immediate safety risk. It is likely that LOD requirements for tests for adulteration scenarios which do not relate to such severe safety risks could be less strict if necessary, if this facilitates method development using cost-effective and portable technologies. The financial value of the oils in question will of course contribute to requirements of the LODs with lower LODs being preferred for oils of higher economic value such as olive oils.
5.2.3. Emerging technologies in the traceability of edible oils
In order to develop methods to detect adulteration in edible oils, Dr Mosely recommends performing non-targeted mass spectrometry applications in the first instance to capture as many metrics as possible. The dataset would ideally comprise thousands of oil samples. Then pattern analysis would be used to determine the key metrics to focus on to identify markers of species origin/oil type. This approach should include chromatography in order to separate and analyse the maximum number of components, and then chromatographic retention time can also be included in the metrics. Use of accurate mass spectrometry and considering peak intensities and peak ratios between components to determine patterns and signatures is Dr Mosely’s recommended initial approach. The aim would be, once the markers have been identified, to transfer the method away from the high-cost high resolution mass spectrometry methods.
Emerging technologies for investigation:
-
Use of small footprint, cheap and robust single quadrupole mass spectrometers setup for direct analysis would provide rapid screening analysis, with suspect samples highlighted for follow-up investigation by a confirmatory technique. Using single quadrupole mass spectrometer removes the capability of controllably fragmenting ions once isolated, but there is the potential that, if required, all ions could be fragmented in the ionisation step prior to mass analysis if the fragmentation pattern proves important in determining markers or signatures.
-
Direct analysis technologies have shown promise, involving minimal sample preparation, easy for non-experts to manage, and with no carryover between samples. Carryover would give false readings, and must be avoided at all costs. Two emerging methods of sample introduction look to be promising ways to prevent this. Direct Analysis in Real Time (DART) and Atmospheric pressure Solids Analysis Probe (ASAP) both show promise for robust measurement of these types of chemical. DART analysis is already showing potential to determine food and illegal drugs.
-
Ion mobility mass spectrometry is a relatively new capability yet has shown promise in related application areas. Here, once the molecules are ionised, they can be separated based on their shape or size prior to measurement. There are challenges here for the study of edible oils due to very small differences in molecular size, but the research has yet to be done.
-
UV photodissociation coupled with high resolution mass spectrometry to investigate the position of fragmentation within a molecule may identify oil-specific markers and signatures.
-
Broader lipidomics studies may prove beneficial to differentiate edible oil types, including analysis of DAGs, TAGs and also phospholipids, ceramides etc. Lipidomics using Fourier Transformation (FT) mass spectrometry including investigations of cis and trans double bonds in lipids during ozonolysis (ozone-induced fragmentation) in mass spectrometry is an interesting area for future focus. This would include possible determination of positions of cis and trans double bonds on saturated acyl chains and tracking their fragmentation patterns to determine if this provides information to differentiate the species of an oil.
-
Dr Mosely recommends lipidomics will be valuable for both discovery of markers and confirmatory testing of samples shown to provide suspect authenticity data to identify species markers.
-
Stable isotope mass spectrometry will feed into the authenticity of some oils to determine geographical origin, which may be relevant, for example when differentiating palm oil from other oils, and may also feed into tracing the authenticity. In the future, rapid low-cost tests involving the analysis of carbon and oxygen isotopes using direct infusion with Fourier transform ion cyclotron resonance (FT-ICR) should be investigated as these may be appropriate to determine geographical origin.
-
Early advances in R&D in this area will drive the instrument manufacturers to rapidly refine technologies to support edible oil authentication and support the curation and updating of reference libraries.
-
It may prove to be beneficial in the future (further data required) to prepare a synthetic oil in the lab with a different number of carbons in the acyl chains to existing edible oils to use as an internal reference standard and spike into samples prior to analysis to assist in quantitation.
Further research is also required to better understand the composition of TAGs in edible oils. The positions of the three fatty acids on the glycerol backbone of the TAG are known as sn-1, sn-2 and sn-3. While it is assumed that the order of these fatty acids is the same across 100% of the TAGs within a pure oil of a given botanical species, Dr Mosely warns that this has not to her knowledge been categorically verified and further research in this area should be confirmed before this premise can form the basis of any future method. This verification can be approached by studying fragmentation patterns during mass spectrometry and comparing these fragmentation patterns with those of synthetic fatty acid standard materials. This work is complex even for pure oils and will be more so for samples containing mixtures of oil species.
5.2.4. Analysis of other oil components
Fatty acid methyl esters (FAMEs) and sterols, are other components of edible oils and are outside of Dr Mosely’s direct area of interest so have not been explored by her. Dr Mosely stated that sterols may perhaps be an easier form of marker of edible oils but may be less useful in quantifying level of adulteration. While DAG and TAG data can be extrapolated to interpret quantity (% adulteration), this is unlikely to be the case with sterols due to ionisation considerations. Sterols may however comprise useful qualitative markers of oil species. Dr Mosely speculated that these components of edible oils may require chemical derivatisation to alter the molecules so that they are better suited to analysis, but that this is linked to inherent challenges when considering the limits of detection and dynamic range.
5.2.5. Future considerations when developing methods to authenticate edible oils
Other considerations during the method development stage will be the reproducibility of oil profiles. For example, a gutter oil originating from a Chinese food outlet may differ a great deal from gutter oil produced by a fish and chip shop. Ideal markers for gutter oil in particular may turn out to be oil degradation products.
Once methods have been developed, there will be consideration of the applicability of methods to cooked oil and processed foods, for example oil found in crisps which have been fried in oil. Degradation studies should be conducted to account for food processing. DAGs and TAGs may prove valuable to determine species specificity after cooking due to degradation profiles.
When extracting oils from final processed products containing meat, it may be possible to determine markers of meat species origin more easily and via lower-cost testing than by current genomic and proteomics methods in a method of combined outputs (measuring both edible oil type plus species of meat).
6. A critical, unbiased literature review to identify current and emerging analytical methods suitable for the analysis of oils
6.1. Status of the edible oil industries, factors affecting those industries and current regulation
Europe is currently the largest producer of olive oil accounting for more than 70% of the world’s production. Non-EU countries are expanding their domestic production and increasing the competitiveness of the global olive oil market. The high price of olive oil, the distinctive sensory profile and its reputation as a healthy source of dietary fats makes olive oil a target for adulteration by illegal blending with other vegetable oils and deliberate mislabelling. The lack of efficient and harmonised analytical methods for detecting edible oil fraud has led to significant weaknesses that are exploited by counterfeiters. As a result, olive oil adulteration for the purpose of financial gain, has become one of the biggest sources of agricultural fraud in the EU.
The financial value of edible oils from different botanical origins can fluctuate, and fluctuations in the value of food commodities can be drivers of food fraud. Often oils of high perceived health benefits carry the highest value, and their availability can impact the value. The market value and availability of various edible oils are subject to fluctuations due to multiple factors, including the inherent value of different botanical oil types, extreme weather events impacting growing seasons, and geopolitical disruptions affecting supply chain logistics. These variables, coupled with the intricacy of the global food supply network, significantly increase the risk of fraud in the edible oil industry. Consequently, the potential for adulteration or substitution of oils becomes a critical concern that must be considered.
Monitoring of authenticity risks is important to protect consumer rights, consumer safety and consumer confidence. Methods must also be in place to protect from safety incidents. Such an example is the incident of Spanish toxic shock syndrome of 1981 which caused the death of over 350 people and permanently affected the health of over 20,000 others when industry-grade rapeseed oil was distributed in the edible oil market (WHO, 2004). The inclusion of industrial mineral oils or gutter oil (recycled food oil) in edible oils also comprises a safety concern linked to the adulteration of edible oils. In terms of the rights and confidence of consumers, foods must be labelled accurately, and methods must be in place to verify these labelling declarations. For instance, procedures should be established to ensure that if consumers choose to buy oil rich in polyunsaturated fatty acids, which are associated with health benefits, it can be verified that the oil has not been replaced with oil containing saturated fatty acids.
Loss of public trust resulting from food crime can have major economic consequences in the short term due to reactions in public behaviour (Macready et al., 2020). Issues regarding the perceived trustworthiness of food labels, and the associated economic impact, can continue in the longer term. For example, the 2013 horsemeat incident is estimated to have cost the UK industry approximately £850 million. Finally, authenticity of edible oils can contribute towards the sustainability targets of food supply companies, for example ensuring that any palm oil included in a product does not originate as a result of deforestation.
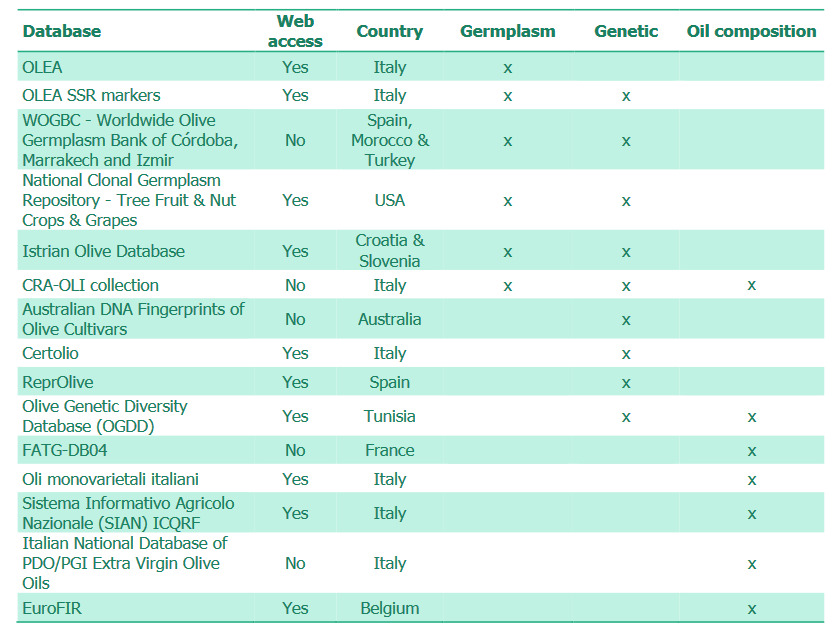
ISO standards relating to methods for testing oilseeds, of which there are 29, are listed under ISO International Classification Standard 67.200.20. Certain of these relate to detection of chemical contaminants, often as a result of environmental contamination. ISO standards relating to methods for testing animal and vegetable fats and oils, of which there are 96, are listed under ISO International Classification Standard 67.200.10. Standards of the International Olive Oil Council are also available. Standards relating particularly to oil authenticity are shown in Table 1a and 1b.
The International Olive Council has a list of standards which covers trade standards of olive oil and pomace oils, testing methods, and organoleptic assessment methods and standards for virgin olive oils. Many of the testing standards overlap with those of ISO.
The UK legislation relating to olive oil composition marketing standards is The Olive Oil (Marketing Standards) Regulations 2014, No. 195. Outside of regulation, International Olive Council (IOC) standards and Codex Alimentarius standards apply. IOC standards only apply for member countries of IOC and official EU methods implement the IOC methods. Codex Alimentarius is an international reference point for food safety that applies to all countries that trade food internationally. However, while EU regulation is mandatory, Codex and IOC standards are not.
Regarding the geographical origin of olive oil, assimilated EU Commission Regulation 2022/2104 applies in the UK, supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012.
The Codex standard for olive oil is Standard for olive oils and olive pomace codex standard 33-1981, adopted in 1981, revision: 1989, 2003, 2015, amendment: 2009, 2013, reviewed in 2017.
Few standardised laboratory-based methods to determine the authenticity of edible oils exist. The main focus on method standardisation has been in relation to olive oils, due to the high value of olive oil which makes it vulnerable to fraud which can impact on the producing nation’s economy. Organoleptic testing by a panel is also available for olive oil (IOC Panel Test, according to EU Regulation 1227/2016) but requires specialist personnel and there is some level of subjectivity to this testing which is far from ideal. As reviewed by Conte et al. (2020), methods available to determine the purity of olive oils can be compromised if certain vegetable oils are present or if method performance or sample extraction/preparation methods require improvement. Many authors have applied various approaches to investigate the authenticity of edible oils of other botanical origins such as rapeseed, sunflower and coconut oil. These approaches include analysis to understand the unique chemical profiles of each botanical origin or the identification of biomarkers using various technologies. Outside of olive oil authenticity, few if any methods have been developed to the stage whereby intra- or inter-laboratory validation studies can be conducted with a future view to develop harmonised methods which would support the food supply industry and related customer trust.
According to the Codex standards (Codex standards for fats and oils from vegetable sources (Codex Alimentarius Commission, 2017)), the authenticity of an oil is confirmed based on the relative proportions of fatty acids present. However, there are not specific ranges provided for the cold-pressed seed oils. There are many cases in which the fatty acids profile of the cold-pressed seed oil and extracted oil is the same (e.g. canola/rapeseed, corn, sunflower, soybean oil (Codex Alimentarius Commission, 2017)), so the use of chromatographic methods for the authentication process would not lead to eloquent results (Neves & Poppi, 2020).
Methods were developed during the Horizon Europe 2020 OLEUM project and standard operating procedures were produced as part of the project to support olive oil authenticity testing. The methods developed aimed to support official testing methods. One such method determined free and esterified sterols by solid phase extraction and GC-FID to identify adulteration of olive oils with other seed oils. The official method already available was focussed on total composition of sterols, whether or not they were free or in the esterified form (ISO 12228, COI/T.20/Doc. No. 10, Reg.(CEE) 2568/1991, All. V). The new method did not replace the official method, but was reported as offering a complementary method which, when used in combination, provided a higher degree of information to support the prevention of food fraud. Another SOP produced during the OLEUM project was a revised analytical approach for Fatty Acid Ethyl Esters (FAEEs) determination by SPE-FID to define the quality grade of virgin olive oil and detect fraudulent mixtures with lower quality oils (virgin, lampante or soft-deodorised oils). The method was validated in-house to provide a quicker testing procedure which also required much lower solvent volumes for extraction compared to the official method for the determination of the content of waxes and fatty acid alkyl esters (as defined in EEC Regulation 2568/91). (ISO 12228, COI/T.20/Doc. No. 10, Reg.(CEE) 2568/1991, All. V). A SPME-GC-MS and a SPME-GC-FID method was also developed during the project to determine volatile compounds in virgin olive oils to determine quality and purity. These methods would require inter-laboratory validation before they can be considered as official methods to understand measurement uncertainty.
There is clearly a requirement to further develop and standardise methods to authenticate edible oils to protect the supply chain. This independent literature review critically assesses the various current and emerging methods available to address the authenticity of edible oils to protect consumer interests. Recommendations are made to future-proof the edible oil industry. The future provision of rapid and reliable point-of-use screening methods to check oil authenticity within the supply chain would be ideal. It is envisaged that more accurate confirmatory methods may also be required to evaluate samples flagged as unsatisfactory or suspect during a rapid point-of-use screening process. Again, these methods should be as accessible as possible to support enforcement (Public Analyst Official Laboratories), with extra consideration to the types of instrumentation available in these labs.
The scope of this review is to consider methods which can be applied to edible oils common to the UK market. The main focus is to detect adulteration of edible oils of one botanical species with that of other species or with non-food grade oils. However, the adulteration of extra virgin olive oil with other olive oils is also considered to a slightly lesser degree. The correct assignment of the geographical origin (GO) of oils is given less focus. While GO testing is very important to the producing nation as adulteration can impact greatly on their economy and there is often a perceived higher quality of oils of certain GOs over others so there is a high level of national support to certify GO, this is in general of lesser consideration to UK customers.
The literature review captured peer-reviewed manuscripts and grey literature published by researchers across the globe, considering manuscripts published between January 2015 and October 2024. The status of methods is discussed, along with any limitations of the technologies, knowledge gaps and challenges. Emerging methods are also evaluated. Recommendations are made to support the edible oil industry going forward.
6.2. Search terms for literature review
A search of the international scientific literature, searched via Science Direct/Web of Science and google scholar, was conducted using the following search terms:
Fatty Acid-Methyl Ester(s) (FAME(s))
Fatty acids
Peroxide value
Triacylglycerol (TAG)
Sterol
DNA
Isotope+ratio
e-tongue
e-nose
Oil AND Food+authentic* OR food+integrity OR food+adulteration OR food+fraud
Oil AND Food AND Trace*
In combination with oil types:
Gutter oil
Palm oil
Rapeseed oil (OSR, canola)
Olive Oil
Extra Virgin Olive Oil (EVOO)
Sunflower Oil
Mineral Oil (MOSH/MOAH)
Coconut Oil
Nut Oil (hazelnut, walnut, ground nut etc)
Vegetable Oil (Soybean, avocado etc)
Seed Oil (Sesame, grape seed etc)
6.3. Spectroscopic techniques applied to edible oil authenticity through analysis of bulk properties
6.3.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR is a widely used technique for the detection of edible oil adulteration. Its ability to detect signals from high molecular weight polymers such as triacylglycerols, heightens its applicability compared to some other techniques. However, the NMR spectrum of edible oils from different botanical sources (such as hazelnut and olive oil) can be very similar and therefore not all types of adulteration can be detected. Where the bulk composition of an oil is characteristic of its source, then NMR is a highly effective way of characterising edible oils. The level of adulteration that can be detected varies depending on the oil type and is usually in the low percentage range. NMR equipment can be expensive and particularly for high resolution instruments. It can also require considerable skill to record and interpret the data in an informative way. The NMR spectrometer can be configured to detect carbon (13C) signals or hydrogen (1H), and studies in the literature have used both approaches. 13C-NMR is intrinsically less sensitive then 1H-NMR but can have better resolution between the NMR signals due to a wider chemical shift range for carbon atoms.
Although some studies have used chemometrics linked to the NMR analysis of a reference dataset (database) this is largely for geographical origin determination and where data trends are sought. NMR is predominantly used to determine the composition of authentic oils and as such does not usually require a database, however authentic reference samples are essential for successful studies.
13C-NMR is of particular use as it provides a large amount of information about the composition of the Mono-, Di, and Tri-acylglycerols (MAGs, DAGs & TAGs) and fatty acids (FAs) in the oils, particularly in relation to structural isomers (Merchak et al., 2016, 2017). It also provides information about the concentration of the low molecular weight components of oils such as sterols.
In an application-focussed study aiming to implement 13C-NMR within a commercial laboratory setting, a database was constructed from samples made up of 81 animal fats and 193 plant fats, together with 20 samples of margarines. Tests were carried out on 81 samples of food products such as biscuits (74) and spreads (7) (Guyader et al., 2018).
In this study, 13C-NMR was used in combination with High Performance Liquid Chromatography (HPLC) to identify the species origin of oils and fats, including processed fats, in commercial food products. Both animal fats and oils were used to construct a database of NMR profiles. The database contained many edible oils from: sunflower (32), rapeseed (also known as canola) (23), olive (20), peanut (17), maize (15), palm (12), grape seed (11), hazelnut (7), coconut (6), argan (5), avocado (5), soybean (5), walnut (5), sesame (3), algae (2), almond (2), copra (2), apricot kernel (2), palm kernel (2), olive wrinkles (2), wheat germ (1), macadamia nut (1), pistachio (1), aniseed (1), rosemary (1), oregano (1), baobab (1), jojoba (1), borage (1), hemp (1) and mustard seed (1).
Animal fats were mainly butter (43 samples). The other animal fats were chicken (1), duck (1), poultry (5), beef (1), lamb (1), pork (7), fish oils (15) and eggs (7). Plant fats were in the form of solids such as margarines (20), cocoa butter (3) or shea butter (1).
The study concluded that in combination, High Performance Liquid Chromatography (HPLC) and NMR, with the application of chemometrics, was able to detect the presence of palm oil in food products down to 5%, improving on the more common fatty acid profiling, which was able to detect 15% of palm oil. The authors also concluded that the 13C-NMR was able to readily distinguish between oils and fats of either plant or animal origin, with adulteration of 5% being readily detected. Several other studies have also focused on using NMR to detect the adulteration of butter with plant oils (de Novais et al., 2024) or non-butter fats (e.g. lard) (Fadzillah et al., 2017). The first of these studies demonstrated that NMR was “unequivocal” in its ability to detect the presence of vegetable oils in butter, whilst also employing stable isotope ration analysis (SIRA) to determine the presence of C3 plant oils. The latter study found that NMR can clearly distinguish between lard and butter and was determined to be a powerful tool for detecting this type of fraud when compared to HPLC.
The majority of studies in the literature have applied NMR to assess the authenticity of olive oil and in some cases compare with more traditional methods of analysis (Sayago et al., 2019). This has largely focussed on geographical origin assessment due to the need to protect national interests in high quality olive oils from countries such as Italy, from products that are perceived to be of lower quality. The intrinsic economic value of products such as Extra Virgin Olive Oil (EVOO) means that there can be a large amount of national support for origin certification for products derived from the producing country and this is often underpinned by using NMR in combination with other techniques and particularly mass spectrometry (MS). The application of the combined use of NMR and MS for the certification of olive oil is extensively reviewed (Calo et al., 2022). They conclude that “both NMR and MS-based approaches represent a mature field where a general validation method for EVOOs geographic origin assessment could be established as a reference recognised procedure.”
Some studies combine the use of NMR with Isotope Ratio Mass Spectrometry (IRMS) and chemical analysis techniques to construct statistical models (Camin et al., 2016). This study showed that Italian olive oils were richer in squalene and unsaturated fatty acids, whereas Tunisian olive oils showed higher δ18O, δ2H, linoleic acid, saturated fatty acids, b-sitosterol, sn-1 and sn-3 diglyceride values. Furthermore, all the Tunisian samples imported were of poor quality, with a K232 nanometre level of oxidation and/or acidity values above the limits established for extra virgin olive oils. They concluded that by combining isotopic data with 1H NMR data using a multivariate statistical approach, a statistical model able to discriminate olive oils from Italy and those imported from Tunisia was obtained, with an optimal differentiation ability of around 98%. Other studies with similar aims applied different technologies in combination with NMR. In one study the TAG content as measured by NMR and LC-MS was highlighted as the key differentiating factor between single origin and multi origin blended EVOOs (Lukic et al., 2020).
Other studies on olive oil have focussed on the olive cultivars used to prepare EVOO. Olive cultivars specific to France and Portugal were distinguished from each other using a combination of 1H and 13C-NMR and Fourier Transform- Mid-infrared spectroscopy (FT-MIR). A high level data fusion approach and chemometrics were required to improve classification models (Maléchaux et al., 2021). Quality parameters such as colour and the elevation of olive groves above sea level have also been correlated with NMR spectral features in building towards a method that can predict oil quality (Merchak et al., 2018).
A paper detailing the use of low-field NMR for the determination of oil composition has also been published (S. H. Wang et al., 2021). This study shows that adulteration of olive oil with corn and soybean oil can be detected using this relatively inexpensive approach. The detection limits calculated using multivariate statistics are between 20 and 30% adulteration of the olive oil.
Whilst fewer in number, other significant studies have used NMR to determine the composition of avocado (Tang et al., 2021), sesame (Kim et al., 2015), grape seed (Tociu et al., 2018) and fish oils (Akanbi & Barrow, 2018). Each reported successful results using NMR to establish oil authenticity, with results being compared to those obtained using, for example, FAMEs and FTIR. These studies establish proof-of-concept for the use of NMR for the detection of edible oil adulteration for these oil types, but further validation studies would be required for these individual studies before application outside of the research and development environment.
6.3.2. Infra-red spectroscopy
Infrared (IR) spectroscopy is a technique used to identify and analyse materials by studying how they interact with infrared light. This interaction gives clues about the chemical structure of a substance because different chemical bonds absorb specific wavelengths. IR spectroscopy is widely used in many fields, including food quality control. It is non-destructive and often rapid. IR spectroscopy often generates characteristic fingerprints that can be related to the composition of an oil and requires an often-extensive reference data set or “database” to compare the fingerprints to. Its main limitation is that the data is almost impossible to interpret, and so a lot of faith is required with this pattern matching technique often being referred to as ‘black box’. Some authors also comment on the lack of transferability of the reference data between instruments as the recorded spectra can be influenced by instrument specification and local factors such as environment.
For oil analysis, IR data is usually analysed using multivariate statistics to produce classification models, which limit the use of the technique for analysing single samples without an extensive reference database. There are some exceptions to this, particularly when exploring the mid infrared (MIR) spectrum, where quantitative results can be obtained. A good example of this is for the detection of total sterols in EVOO (Özdemir et al., 2018). Whilst the method used (in this case FTIR) was robust for detecting total sterols, the authors highlighted the limitation of the approach to provide more detail about changes to the individual levels of sterols in a range of Turkish EVOOs. The method was also able to detect changes to the fatty acid composition of the oils particularly where fatty acids were grouped according to their saturation status.
Types of Infrared Spectroscopy
There are three main regions of the infrared spectrum, each used for different purposes (summarised in Table 2):
-
Near Infrared (NIR) Spectroscopy:
-
Wavelength Range: 0.8–2.5 µm.
-
Key Features: NIR is mainly used to detect overtones and combinations of fundamental vibrations. It is less sensitive than other IR regions but allows for fast, non-destructive analysis of materials. Some studies have shown its use for detecting specific components in oil such as water (Azizian et al., 2021) whilst others have focused on geographical origin determination (Laroussi-Mezghani et al., 2015) Persuric et al., 2018).
-
Application to Food: In edible oils, NIR can quickly measure moisture, fatty acid content, and other components such as sterols in pumpkin oil which can be used to detect adulteration with sunflower oil (Balbino et al., 2022). It is particularly useful for checking the quality of large batches of oil without destroying samples.
-
-
Mid Infrared (MIR) Spectroscopy:
-
Wavelength Range: 2.5–25 µm.
-
Key Features: MIR is highly sensitive and can provide detailed information about molecular structure. It is the most commonly used region for identifying specific chemical bonds. It has also been used in applications that are highly relevant to this review, such as the detection of palm oil in sunflower oil (Srinath et al., 2022). This study showed attenuated total reflectance (ATR)-based MIR spectroscopy’s potential for the detection of palm oil adulteration in sunflower oil at a minimum of 5% adulteration.
-
Application to Food: MIR can be used to detect adulteration by identifying the unique fingerprint of different oils. This helps ensure the authenticity of high-quality edible oils.
-
-
Far Infrared (FIR) Spectroscopy:
-
Wavelength Range: 25–1,000 µm.
-
Key Features: FIR explores lower-energy vibrations, including lattice vibrations in solids. It is less commonly used in food analysis due to its focus on bulk material properties rather than specific chemical structures.
-
-
Fourier Transform Infrared (FTIR) Spectroscopy:
-
FTIR is not a specific region but a method that improves IR spectroscopy. Instead of measuring light absorption at each wavelength individually, it uses a mathematical process called Fourier Transform to measure all wavelengths simultaneously. This makes the analysis faster and more precise.
-
Application to Food: FTIR is highly effective for analysing edible oils and has been used extensively in combination with multivariate statistics to classify oils by, vintage, geographical origin and according to quality parameters (Zaroual et al., 2021b). It can rapidly detect small amounts of adulterants, assess oxidative stability, and measure the levels of different fatty acids. For example, it can identify if an olive oil sample has been diluted with sunflower oil by comparing their characteristic spectra.
-
Examples in Edible Oil Analysis
-
Quality Control: IR spectroscopy can assess whether an oil meets industry standards by measuring its chemical composition, such as the ratio of saturated to unsaturated fats. It can also be used to assess the purity of the cultivars used to produce EVOO (Abdallah et al., 2016) (Lamas et al., 2021).
-
Authenticity Testing: Premium oils such as EVOO are often expensive, and adulteration is a common problem. IR spectroscopy can sometimes confirm their purity by comparing the IR spectrum of a sample with that of authentic oils. This has been shown to be true for a range of other oil types such as camellia oil that had been adulterated with hazelnut, soybean, corn and palm oil (He & Lei, 2020). Although not extensively covered here, this review has identified papers where FTIR in particular is used for the determination of geographical origin of oil such as Argan oil from Morocco (Kharbach et al., 2017) and NIR has been used in a small study as a complementary technique to mass spectrometry for geographical origin (Persuric et al., 2018) .
-
Detecting Degradation: Oxidation of oils over time affects their quality and taste. IR spectroscopy can monitor the level of oxidation products, helping producers ensure their oils are fresh (Liu et al., 2020).
FTIR in particular has been shown to be able to distinguish between different oil types with a range of oils being assessed. For example, (Ladan & Glavac, 2022) distinguished between 17 oil types which were: cranberry, elderberry, borage, blackcurrant, raspberry, black mustard, walnut, sea buckthorn, evening primrose, rosehip, chia, perilla, black cumin, sacha inchi, kiwi, hemp, and linseed oil. They showed that certain components of the oils were useful for classification using a multivariate model. a-linolenic acid and linoleic acid contents were found to be particularly well correlated to oil type. However, little data is presented to show the use of the models for detecting adulteration with other oil types.
IR spectroscopy has also been used to detect the adulteration of Virgin Olive Oil (VOO), with both soft-deodorised EVOO and with refined oils. It was found that the heat treatment needed for soft deodorisation changed the composition of the oil including: anisidine value, pyropheophytin, 1,2-diacylglycerols, total polar compounds and monomeric oxidized triacylglycerols (Gertz et al., 2020).
In a preliminary study, an in-house validation of Visible and Near Infrared Spectroscopy was performed to distinguish between EVOO and VOO (Garrido-Cuevas et al., 2024). A total of 161 samples of olive oil of three different categories (EVOO, VOO and lampante olive oil (LOO)) were analysed. The model yielded correct classification values of 82.35 % for EVOO and 66.67 % for VOO. These results confirmed that the technology combination may be used to provide rapid, non-destructive preliminary screening of olive oil samples for categorization of samples. The method would form the basis of a screening test, after which unsatisfactory samples could be analysed by official analytical methods. The application of this technology has the future potential to develop analyses using point-of-use, portable devices. The authors highlight that this spectroscopic approach seems to perform at least in line with, if not better than, methods based on chromatography. This carries the benefit that spectroscopic methods tend to be faster, cheaper and require non-experts, using lower cost instrumentation and negating the need for environmentally toxic reagents. The authors called for the organisation of future studies involving this approach by IOC, with a broader and more numerous sample set compared to this preliminary study.
In a novel application, FTIR was used to determine the frying oil type used to make crisps (potato chips) the authors noted that this could lead to test to assess the source of oil used in cooking which could be used at point-of-contact (Aykas & Rodriguez-Saona, 2016) but more work would be required to develop this into a “real-world” application. This would, for example, include studying the effect on the data of different cooking times and temperatures. A major advantage of the technique is its potential for use in the field to support ongoing fraud investigations and this has been reported during an incident relating to olive oil adulteration in Spain (Santos et al., 2020).
6.3.3. Other spectroscopies
6.3.3.1. Raman spectroscopy
Raman spectroscopy is a technique used to study the composition of substances by applying a laser. When the laser interacts with the molecules in the sample, most of the light bounces back unchanged, however, a small amount of light scatters in a different way (this is called Raman scattering) and it provides valuable information about the molecular structure of the substance. A review of the literature by Talib et al. (Talib et al., 2024) aimed at detection of fraud and contaminants suggested the use of Raman spectroscopy as a screening tool followed by mass spectrometry as a possible approach. However, this literature review was not supported by testing to substantiate the suggested approach. Raman spectroscopy, in this case Spatially Offset Raman Spectroscopy (SORS), has been used successfully in a study to differentiate olive oils and pomace from compositionally similar high oleic acid content sunflower oils (Jimenez-Hernandez et al., 2024).
Advantages of Raman spectroscopy:
-
It is non-destructive, meaning the sample remains unchanged.
-
It requires very little preparation of the sample.
-
It works well for liquids, solids, and powders.
-
It can be used on samples inside containers like glass, which is especially helpful for food testing.
Disadvantages of Raman spectroscopy:
-
Raman is an inherently insensitive technique with low information content and used mainly to investigate the bulk composition of products.
-
It can also change the composition of the sample due to heating whilst making the measurement.
In summary, Raman has been used infrequently for oil analysis due to some major limitations of the technique which are discussed to some extent by (Aykas et al., 2020) and may be better suited to providing supporting information to other techniques largely due to the complex nature of natural food products such as oil and honey, and the limited resolution of the technique (Liu et al., 2020).
6.3.3.2. Terahertz spectroscopy
The Terahertz (THz) spectrum refers to electromagnetic waves with a frequency from 0.1 to 10 THz, between microwaves and infrared waves. The skeleton vibration, dipole rotation, and vibration transition of most macromolecules in organic substances and the weak interaction between them can be reflected in the THz spectrum. In their review of research progress of Terahertz spectroscopy, Zhang and colleagues (Z. X. Zhang et al., 2021) described the THz technology as possessing great potential in food adulteration detection. However, this manuscript (other than the abstract) is only available in Chinese so could not be verified.
6.3.3.3. Electron Paramagnetic Resonance spectroscopy (EPR)
Another area which is relevant to edible oil authenticity involves Electron Paramagnetic Resonance spectroscopy (EPR), also known as Electron Spin Resonance spectroscopy (ESR). Although there is no reported use of this technique for authenticating edible oils, it has been used to study oil quality pertaining to oxidation and is therefore relevant to the detection of aged oils, over processed oils, or gutter oils. Analysis with EPR allows the monitoring of the proportion of fatty acid types present (increase of saturated and decrease of polyunsaturated fatty acids), monitoring of newly formed aldehydes and peroxides and measuring of oil oxidative stability (Castejón et al., 2017) (Jiang et al., 2020). Each of these aspects which can be applied as indicators of deep-fat frying and could therefore be used to determine the presence of gutter oil in edible oil.
6.3.4. Conclusions to application of spectroscopy technologies
Spectroscopies are very useful tools for the detection of oil adulteration. NMR spectroscopy in particularly is a well-established way of detecting edible oils that have been adulterated with oil from other botanical sources or with oil products that are not of sufficient quality. With a suitable reference sample collection, this technology can be used to identify signals that do not arise from the intended oil source. Unlike many other natural products such as honey, oil is often highly refined and usually from one botanical source frequently from a well-defined location, so biological and processing variation is often quite low for a particular oil type. This means that there is less reliance on NMR databases and the data can be interpreted directly from the 13C and 1H NMR spectra.
Where technologies can be “black box” such as FTIR and Raman spectroscopy, there is a need for a reference dataset to compare spectra to. The number of samples needed in the reference data set will be determined by the scope of the issue being addressed. As the origin (botanical and geographical) of oils is usually declared, then reference data should be relevant to the oil source. The major advantage of IR is the potential for it to be portable and therefore used in-field as a screening technology to potentially identify suspicious oils. Raman spectroscopy has the added advantage of being able to work through the bottle and is therefore considered non-destructive, even if it does heat the sample when making the measurement. Finally, EPR offers potential to detect gutter oils.
In summary, NMR is ready for use for detecting oil adulteration. FTIR is probably the most promising of the field-based technologies for screening oils if a good reference dataset is available. There have been far fewer studies using other spectroscopies such as Raman and EPR which could be explored further. There are now, for example, cheaper handheld Raman instruments on the market which may not have been trialled for oil authentication. It would be interesting to perform further studies to better-understand the applicability of these spectroscopic methods.
6.4. Separation science technologies applied to edible oil authentication
While both GC-MS (Gas Chromatography-Mass Spectrometry) and GC-FID (Gas Chromatography-Flame Ionization Detector) share chromatographic separation capability, the key difference lies in their detection methods. GC-MS provides a mass spectrum which can be interpreted to provide detailed structural information about a compound, identifying multiple known or unknown compounds in complex mixtures. GC-FID, a lower cost technology, is primarily used for quantitative analysis of known compounds in relatively simple mixtures. GC-MS offers high sensitivity while GC-FID offers medium sensitivity. For oil analysis, GC-MS offers the benefit of not needing standards in order that each compound is identified as libraries are available for this. GC-FID can be used for quantitation if a reference standard material is available for just some of the analytes.
Regarding mass spectrometry technologies, while MS, coupled to gas or liquid chromatography, provides a single mass measurement and is suitable to identify a compound in simple mixtures based on mass and retention time, MS/MS involves two mass analysers coupled together. This coupling of mass analysers allows a fragmentation step which enables the identification of molecules based on their fragment ion masses and can reveal structural detail to identify multiple compounds. It is advantageous as it can be applied to complex mixtures. MS/MS generally offers increased specificity and sensitivity compared to MS. Mass spectrometry technologies offer high sensitivity solutions.
Liquid chromatographers can be coupled to different detectors such as ultraviolet (UV) and charged aerosol detector (CAD). UV selects a specific wavelength and can identify and quantify separate components in a mixture based on the retention time and comparison of UV spectra with reference compounds. CAD is appropriate when an analyte does not have a chromophore functional group and cannot be detected by UV-Visible spectrophotometry (UV-Vis), including semi-volatile, or polar compounds like lipids, carbohydrates, and surfactants. CAD and UV detection offer low-to-moderate sensitivity. The details above are summarised in Table 3.
6.4.1. Molecular characterisation: Analysis of fatty acids and other volatile compounds for edible oil authenticity by Gas Chromatography Mass Spectrometry
Authenticity of edible oils is important to country’s economies and in terms of consumer safety and trust and is therefore regulated by standards to determine if the oil is pure or of an appropriate quality. Some variables found in different edible oils include region, harvest time, cultivar, grade of fruit, whole fruit oils or mesocarp oil and these variables can be identified as a fingerprint for different qualities of oil. Chemical parameters analysed to determine the fingerprint of oils are compounds which are represented in 95% of edible oils: fatty acids (FAs), phytosterols, tocopherols, tocotrienols, mono-, di- and triacyclglycerols (TAGs). The principal olive oil classification in terms of quality is related to the acidity and organoleptic characteristics, which are directly associated with the olive oil processing (Peixoto, 1973). FA composition of EVOO demonstrates the fruit quality used to produce the oil and if the final oil product has been stored correctly. FA composition varies depending on the cultivar, fruit maturity, altitude, climate as well as other factors (Olive Oil Source, 2017). EVOO contains more monounsaturated fatty acids (MUFAs), such as oleic acid than polyunsaturated acids (PUFAs) such as linoleic and linolenic acids and is therefore more resistant to oxidation. The major FAs in EVOO are: oleic acid (18:1n-9); a mono-unsaturated omega-9 FA representing from 55 to 83% of total oil composition, linoleic acid (18:2n-6); a polyunsaturated omega-6 FA, representing from 2.5 to 21% of total oil composition, palmitic acid (16:0); a saturated FA representing from 7.5 to 20% of total oil composition, stearic acid (18:0); a saturated FA representing 0.5 to 5.0% of total oil composition and α-linolenic acid (18:3n-3); a polyunsaturated omega-3 FA representing < 1.0% of oil composition (International Olive Council (IOC), 2016).
A wide range of scientific separation and detection methods are applied to analyse these chemical parameters. Generally, gas chromatography (GC) is more suitable for the analysis of smaller, volatile compounds (VOCs), such as FA (which must be converted into FAMEs, hydrocarbons, tocopherols, sterols and triterpenic alcohols while LC is more suitable for larger and less/non-volatile compounds such as phenols, secoiridoids, flavonoids, lignans and triterpenic acids. This differentiation is not absolute as some compounds such as flavonoids can also be analysed by GC when derivatised. GC coupled with an MS detector is a popular instrumentation technique used for analysing FAs, TAGs, VOCs and phytosterols. The major advantage of this detector is that it allows molecular level identification of the compounds based on mass-to-charge (m/z) ratios and relative abundances of the molecular and fragment ions, arising from electron ionisation (EI). Tentative compound identification from a commercial MS library such as that of the National Institute of Standards and Technology, U.S. Department of Commerce (NIST) and Wiley Registry, Wiley Science Solutions can be used to reliably suggest compound identification by comparison to sample mass spectrum (Mota et al., 2021), (Mansur et al., 2018). Targeted compound identification can also be carried out by GC-MS with the use of analytical standards and individual methods for the different classes of compounds (Mansur et al., 2018).
Mass spectrometry (MS) provides a single mass measurement and is suitable to identify a compound in simple mixtures. MS/MS involves two mass analysers coupled together to allow a fragmentation step which enables the identification of molecules based on their fragment ion fingerprint and can reveal structural detail to identify multiple molecules. It is advantageous as it can be applied to complex mixtures.
Gas Chromatography Flame Ionisation Detection (GC-FID), a lower cost technology compared to mass spectrometry, provides a signal proportional to the amount of organic material present and is primarily used for quantitative analysis of known compounds in relatively simple mixtures. While it lacks the ability to definitively identify molecules, sensitivity tends to be higher than many routine GC-MS approaches.
Combinations of clustering techniques such as principal component analysis (PCA), linear discriminant analysis (LDA), canonical discriminant analysis (CDA) or partial least-squares regression (PLSR) analysis are used to differentiate the markers by edible oil type (sunflower oil, olive oil, sesame seed oil etc) either qualitatively (Xing et al., 2019) and (Mansur et al., 2018) or quantitatively (Heidari et al., 2020), (Merchak et al., 2018), (Mansur et al., 2018) (Pastor et al., 2019). A review on quality and authentication of seed and vegetable oils (including processed oils) by analysing mono-di and triacylglycerols, FAs, phytosterols, tocopherols and tocotrienols by both one-dimensional (1D) and multidimensional gas chromatography (MDGC) highlighted various classes of compounds as markers for adulteration. For this work the GC-MS was operated in Atmospheric-Pressure Chemical Ionisation (APCI) mode, to preserve the molecular ion information. Fifty-eight compounds were identified FA (38%), sterols (31%) and secoiridoids (17%) (Mota et al., 2021).
6.4.1.1. Application of large datasets of authentic materials
Large datasets generated from testing samples, ideally with ensured authenticity, underpin GC analyses to successfully determine adulteration in a range of edible oils. Successful methods included whole fruit as well as only the mesocarp, different qualities of fruit, region that the fruit was grown in, harvest time (Green & Wang, 2023), and different levels of erucic acid in rapeseed oil (Xing et al., 2019) within the appropriate methods. Comprehensive studies such as Green and Wang (2023) used large datasets of authentic samples and compared them to the current proposed Codex Alimentarius standard for FA profiles.
In 2016, 20 partners from 15 countries with expertise in food analysis, food legislation, industrial equipment engineering and bioinformatics all formed a consortium. The aim of this four-year Horizon 2020 project, OLEUM, was to improve existing, and develop new, analytical methods for detecting olive oil fraud by identifying novel analytical markers for detecting illegal blends, measuring olive oil freshness and monitoring compliance with labelled geographical origin. GC-MS methods for determining FAs and VOCs, mainly GC-FID-MS, SPE/GC-FID-MS, SPME-GC-FID-MS and SPME-GC-MS, were developed/improved and determined as successful analytical methods for detecting adulteration and freshness in olive oil samples (Conte et al., 2020 and OLEUM, 2023).
During the course of the EU Horizon 2020 OLEUM project, a method underwent an inter-laboratory trial involving 20 participant laboratories (García-González et al., 2023). This study built on an earlier small inter-laboratory study to quantify volatile markers in virgin olive oil to assess quality grade (Aparicio-Ruiz et al., 2022, 2023). This method was developed to provide instrument-based support for organoleptic panel testing and is based on quantitative detection of volatiles by SPME-GC with either, flame ionization detection or and mass spectrometry, depending on the instrument available in each participant lab. The method using FID detector provided better results in terms of reproducibility than the method using MS. The repeatability relative standard deviation values were significantly lower for SPME-GC-FID in certain compounds (octane, ethyl acetate, ethanol, 3-methyl-1-butanol, 6-methyl-5-hepten-2-one, acetic acid, propanoic acid). GC FID technology tends to be available in a higher number of testing labs compared to the higher cost GC-MS. The GC standard methods approved by IOC and EU are mostly based on FID detector. Nonetheless, the method using the MS detector provides the clear advantage of a confirmed identification and often permitted quantification during this study. The authors stated that, in laboratories where both detectors are available, an optimum approach is to use MS for accurate identification (or confirmations, e.g., in the case of overlapping of peaks) and carry out routine analyses using GC-FID. This strategy fits with the logic of analytical sustainability, that is, to carry out the maximum number of analyses at a lower cost.
Past studies have focused mainly on FA profiles rather than sterols and only focussed on some of the variables that can impact oil (e.g. growing region), however Green and Wang (Green & Wang, 2023) successfully tested 68 authentic samples of avocado oil of different quality for each of the variables in both whole fruit and mesocarp only, from different growers at different time points by GC-FID-MS. Green and Wang (2023) concluded that the Codex Alimentarius standard (REP22/FO) (Codex Alimentarius Commission, 2021) adequately accounts for the natural variation of FAs that occur in avocado oil in both the whole fruit and the mesocarp and is not effected by the quality of the fruit, therefore potentially helping reduce food waste as the poorer quality whole fruits can still be used for oil production. Da Silveira and co-workers (da Silveira et al., 2017) also used GC-FID-MS to develop a rapid, innovative, precise, and efficient method to detect levels as low as 1% of soybean oil in EVOO adulterated oil. Thirty-nine sample of EVOO, commercial blends of soybean oil and EVOO, EVOO with international certificate and refined soybean oil were analysed. The EVOO was intentionally adulterated with soybean oil in five replicates at different levels (1, 5, 10, 20, 50, 70, 90% (v/v)) to test the developed method. A total of 17 FAs were determined and the FA profiles for all the samples disagreed with the label on the respective sample, apart from the standard oil. The two commercial blends of oils and one of the EVOO sample showed FAs compositions for a soybean oil in comparison to the standard oil and therefore, could be a characterisation of possible adulteration. The low relative standard deviation achieved from the intentionally adulterated EVOO with soybean oil at different percentages, along with the rapidity and simplicity of the method allows this method to be applied as routine analysis in industry. Merchak and co-workers (Merchak et al., 2018) validated a previously developed method for FAs by Nuclear Magnetic Resonance (1H NMR) and Insensitive Nuclei Enhanced by Polarisation Transfer (13C- INEPT) with a GC-FID-MS method. A training set of 36 authentic green and black Lebanese olive samples was collected from different regions and altitudes, crushed and ground to a paste. Oil samples could be classified according to the altitude of the olive field and the colour of the olives. The statistical correlations between the GC and 13C-INEPT methods fitted perfectly for FAs found in olive oil. FAs, mainly linolenic acid, erucic acid and behenic acid, by GC-FID-MS were analysed by Xing et al. (Xing et al., 2019) for detecting adulteration of 882 samples of sesame oil (73 samples) with blends of four vegetable oils; rapeseed seeds (57), soybean seeds (103), sunflower oil (11 brands) and maize oil (14 brands). Seeds were all purchased from main producing areas in China with different geographical origin and with high and low erucic acid. The oils were purchased from local supermarkets in China. Each sesame oil was also mixed at percentages (0, 5, 10, 20, 30, 40 and 50%) with other oils. Although the parameters calculated by the FA composition were simple and effective in distinguishing between the pure form of the different oils, even from different origins, with the LDA accuracies obtained between 97.27 and 100%, this method could only be used for qualitative analysis due to misclassification of the results for some of the adulterated oils such as sesame oils with maize oils and sunflower oils. For the results to be successfully quantified a partial least squares regression model of the GC-FID-MS FAs data was required. The training set data was validated by 1000 runs of k-fold (k=10) cross validation. The results indicated the model was well-established and accurately predicted oil adulteration.
GC-MS was a technique used by Heidari et al. (Heidari et al., 2020) to quantify animal fat (lard) adulterations in vegetable oil by FAMEs using the Wiley 2007 library from the top three library hits with a >95% match. Averaged data (n=3) for 13 olive oils (12 real samples and 1 standard), 4 lards (3 real and 1 standard) from a range of countries (Spain, Italy, Iran, Kuwait, England, Armenia and France) were calculated and reported using their confidence intervals at the 95% confidence level. The FAME methyl behenate, was only present in olive oil and methyl myristate only present in lard samples. The four most abundant FAMES in both olive oil and lard were methyl oleate, methyl palmitate, methyl linoleate and methyl stearate and these could all be detected in the binary admixtures of lard and olive oil at the various percentage concentrations (5-75%). This method was validated, recoveries and relative standard deviation (%) were calculated (intraday and interday, n=3) to determine the accuracy and precision of the method. Although this study was not carried out on a large data set, the results of the proposed method suggest that it can be used to investigate the authenticity of various animal fats and vegetable oils to detect adulterations using a targeted method for the four distinctive FAMEs markers. A rapid qualitative screening approach using FAMEs to determine the botanical origin of commercial samples from various edible vegetable oils by ion current (IC) GC-MS with NIST14 and WILEY7 libraries (90% match) was developed (Pastor et al., 2019). This simple sample extraction and derivatisation within the injector of the GC instrument analysed 59 samples from 17 different plant species, of which 29 were certified including cold-pressed (21), virgin (7) and refined (1) and 30 were non-certified cold-pressed oils. Pastor et al. claim this rapid screening approach shows a high potential to confirm the botanical origin of expensive and valuable vegetable oil products and determine possible adulterations in the production process and sale of commercial vegetable oils. There was no quality control or validation data present in the manuscript on which to base further judgement.
Purcaro and co-workers (Purcaro et al., 2016) developed a method that investigated the minor compounds (alcohols, sterols etc) which represents less than 5% of the oil composition, as this fraction provides specific information about the identity of an oil Purcaro et al. (2016) generated a quantitative and qualitative unique two-dimensional chromatogram to be used as a chemical fingerprint by using a comprehensive GC system coupled with a simultaneous dual FID detector. Purcaro and co-workers stated that the characteristic 2D plots obtained from derivatisation step prior to the GC x GC-FID/MS was highly informative and could be used to monitor the identity of the quantified compound to avoid potential bias. However, a more detailed investigation comparing these results to those of the more traditional methodologies would be required.
6.4.1.2. Final remarks and conclusions on the suitability of gas chromatography to characterise compounds in edible oil authenticity
Gas chromatography and the related analysis of volatile compounds is a well-used approach to support authentication of olive oils, particularly in relation to olive oil quality. GC-FID technologies tend to be available in testing laboratories, more so than GC-MS. A certain amount of data is available to differentiate olive oil from other botanical species. Further research is required to understand whether methods to determine volatile organic compounds can also be successfully applied to differentiate other oil botanical species, especially in a quantitative manner, this being necessary to investigate deliberate adulteration.
6.4.2. Molecular characterisation: Analysis of triacylglycerols to determine authenticity of edible oils
Analysis of triacylglycerols (TAGs) in the detection of food fraud in edible oils is also discussed in Consultation 2.
Triacylglycerols (TAGs) are the main component of edible plant oils and can be used to determine their authenticity. TAGs are triesters composed of three fatty acids esterified to a glycerol backbone. The FA and TAG distribution has been suggested as the key indicator of edible oil or fat adulteration (Fasciotti & Netto, 2010; Hasan, 2010; Salghi et al., 2014; Tu et al., 2016).
Analysis of triacylglycerols (TAGs) is considered one of the most important issues in the study of fats and oils (Ruiz-Gutierrez & Barron, 1995). TAGs can be differentiated by the number of carbon atoms, number of double bonds, type and stereospecific position of fatty acids on the glyceryl moiety, and also the number, position, and configuration of double bonds in the acyl chains (Sompila et al., 2017). TAGs analysis has received much attention and has been used for various essential applications. This has included investigations into food fraud and adulteration, especially in edible oils (Barison et al., 2010; Bosque-Sendra et al., 2012; Fasciotti & Netto, 2010; Green et al., 2020; Lisa et al., 2009; Marekov et al., 2010; Tsimidou et al., 1987; Xing et al., 2019).
Researchers into edible oil authenticity are increasingly turning to TAG analysis to determine edible oil quality and authenticity. TAGs can be analysed using a range of techniques including GC-FID, GC-MS, LC and LC-MS, as discussed below.
6.4.2.1. Analysis of TAGs by LC coupled to mass spectrometry
The analysis of TAGs to determine quality of edible oils is not a new concept. TAGs in their oxidised forms such as triacylglycerol hydroperoxide, can be analysed by LC-MS to trace quality loss and toxicities due to oxidation (Kato et al., 2018). The analysis of TAGs, (trilinolein, and then Delta ECN42) is recognized as a marker for the presence of extraneous oils in EVOO according to the IOC official method for Equivalent Carbon Number ECN 42 analysis.
Perhaps more applicable to this review, (da Silveira et al., 2017) developed a direct infusion ESI-MS method which used a TAG as a natural lipid marker of soybean oil adulteration of EVOO. The method was able to detect less than 1% soybean oil in EVOO and shows great promise as this adulteration is attractive to fraudsters. Commercial samples were analysed and the addition of soybean oil was found to be common. The authors concluded that “the powerful analytical method proposed could be applied as routine analysis by control organizations, as well as food industries, considering its pronounced advantages; simplicity, rapidity, elevated detectability and minor amounts of sample and solvent consumed.”
Technology advances include the development of ambient mass spectrometry (dos Santos et al., 2016) used easy ambient sonic-spray ionization mass spectrometry to determine the TAG profiles of a range of exotic oils to determine their suitability as food and fuels. The TAG profiles of the following oils were investigated: Jatropha curcas, Bombacopsis glabra, Capparis flexuosa, Siparuna guianensis, Moringa oleifera, Hibiscus tiliaceus, Virola bicuhyba, Pouteria caimito and Syagrus coronata. The TAGs indicated that some of the oils contain mainly short length fatty acids and so were suitable for fuel production, whereas others had TAG profiles similar to palm or soybean oil and may be better for food use. The authors also highlighted that the method was capable of detecting some polyphenols as well as TAGs and fatty acids. In a similar discovery study, the same method was also used to distinguish between species of Salmon based on their oil profiles and specifically PUFA in TAGs (Maluly et al., 2019).
Other authors who have highlighted the potential of TAG profiling in edible oil fingerprinting included Arena et al. (2021) who applied TAG fingerprinting to borage seed oil. Kozub et al. (2023) applied LC-Q-ToF MS to identify TAG and DAG lipid markers to monitor adulteration of sunflower, rapeseed and soybean oils with camelina, hemp and flax oils, due to their different TAG and DAG profiles. Six TAG and DAG markers could identify their addition to rapeseed, soybean or sunflower oils. While the authors acknowledge that further research is needed to validate discrimination based on identified TAG and DAG lipid markers, they conjecture that oil-specific marker panels will allow the development of simple, fast, and robust targeted methods for oil quality assurance, suitable for future implementation in food control laboratories for routine oil authenticity testing. Other authors have highlighted the benefits of TAGs analysis to authenticate oils. Wei and co-workers (Wei et al., 2023) also reported the strong potential of TAGs analysis to authenticate edible oils (Wei et al., 2023; J.-J. Zhang et al., 2023). Wei et al demonstrated identification of adulteration of olive oil with soybean oils, rapeseed oils or camellia oils at concentrations down to 2%.
Dong and co-workers (Dong et al., 2015) used a complex combination of 2D-LC coupled with APCI-MS to study the TAG composition of peanut oils. In this speculative study, 12 samples were analysed and the technique demonstrated that the TAG profiles of high-oleic peanut oil were clearly different from those of normal peanut oils. High-oleic and normal peanut oils had different profiles mainly in the contents of the TAGs oleic-oleic-oleic, palmitic-oleic-linoleic and 1,3-Dioleoyl-2-palmitoylglycerol . The authors concluded that “this finding provided theoretical foundation for detecting the adulteration of edible oils and analysing the nutrition and function of high-oleic peanut oils.”
Other studies have extensively used chemometrics to classify oil samples and detect adulteration using the TAG profiles. Triacylglycerol profiles of edible oils were analysed by Atmospheric Pressure Chemical Ionization Source (APCI-IT) with MS3 ion trap mass spectrometry in (X. P. Wang et al., 2019). All triacylglycerol compounds were quantified by normalization of the chromatographic peak area in selected reaction monitoring (SRM) mode based on MS3 fragment ion pairs, which were used to eliminate interference from the isobaric species of triacylglycerol. The results of hierarchical cluster analysis (HCA) and random forest (RF) classification algorithms indicated that; peanut, soybean, sesame, sunflower seed, and linseed oils are completely classified into five groups based on their triacylglycerol profiles. A recursive support vector machine (R-SVM) discriminant model was established, which successfully identified adulteration of high-priced oil with cheaper edible oils at a concentration of as low as 4% with an accuracy of 93.7%.
6.4.2.2. Analysis of TAGs by LC (without mass spectrometry)
Liquid chromatography offers the opportunity to identify and quantify components in simple mixtures. Green and colleagues applied PCA analysis to data of 11 TAGs quantified in oils by ultra-high pressure liquid chromatography (UHPLC) with charged aerosol detection (CAD) as well as for five common olive oil adulterants including high-oleic sunflower, high oleic safflower, canola, soybean, and grapeseed oils (Green et al., 2020). These oils were chosen based on the likelihood of use as adulterants in EVOO. While this was a small study on a limited dataset, differentiation of oil types, PCA analysis demonstrated clustering of individual botanical types. The method was shown to be rapid compared with traditional approaches and could detect a range of common oils in EVOO with a detection limit of 10% or below depending on the oil type. For oil mixtures, it was determined that adulteration of EVOO could be distinguished at a level of 5% for grapeseed and high-oleic sunflower oils and 10% for canola, soybean, and high-oleic safflower oils. In each case, the best separation on a PCA plot was achieved using three TAGs with trilinolein always being one of the three.
In 2023, extending their previous work on olive oil authenticity, Green and Wang investigated the purity of avocado oil using triacylglyceride analysis combined with PCA (Green & Wang, 2023). The method showed good promise, being as accurate as the established standard methods for fatty acids and sterols (REP22/FO) (Codex Alimentarius Commission, 2021).
In a study by Guissous et al., differences in triacylglycerol and tocopherol (analysed using HPLC) and fatty acids and squalene (measured by GC-FID) compositions were demonstrated between 8 varieties of virgin olive oils (Aberkane, Aguenaou, Aharoun, Aimel, Bouchouk Guergour, Bouichret, Chemlal, and Sigoise) from Petite Kabylie area, northeastern Algeria. Fatty acid and triacylglycerol morphotypes characterized each variety (Guissous et al., 2018).
6.4.2.3. Analysis of TAGs by GC with mass spectrometry
Until recently, the use of TAGs to authenticate oils has been limited by the methods available for their detection. Historically, stereospecific enzymes have been used to cleave FAs from the glycerol backbone to reveal the positional information of the TAGs when subsequently analysed for FAMEs. (Blasi et al., 2019) used an enzymic method to cleave the fatty acids from the glycerol backbone to determine the positional isomers of TAGs in monoculture EVOO. These data were combined with SPE-GC-MS profiles of volatile components of the oil and were found to be able to discriminate between different monoculture Italian EVOO.
Recent advances in mass spectrometry have enabled the direct stereoisomers of TAGs to be distinguished. (Fasciotti et al., 2020) investigated exotic oils from the Amazon rain forest looking at their suitability for use in foods, medicines and as cosmetics. Using GC-ESI-HRMS/MS over 70 different TAGs were identified in each oil and the profiles compared to common edible oils such as soybean, corn, coconut, and olive oil. The study generated good data, but remained speculative about future applications suggesting that the data created would help to guide the use of Amazonian oils to optimise the use of resources and prevent fraud.
6.4.2.4. Conclusions to the application of TAGs analysis to edible oil authenticity
TAGs analysis shows much potential for the accurate, sensitive and robust determination of adulteration issues in edible oils. Applying chromatographic separation approaches, many FAs and TAGs compounds, including minor components in most cases, can be used as variables for statistical analyses. The detection of adulteration can be further improved by applying a chromatographic approach combined with mass spectrometry (such as LC-MS) since the efficient separation by chromatography and exact identification by mass spectrometry enables more comprehensive analysis, in the sense that it enables the determination of intact TAGs, allowing for the evaluation of potential variations in the TAG distributions. Indeed, the majority of recent methods to determine TAGs involve LC-MS.
Information regarding the application of TAGs analysis for the detection of oil adulteration can be found in Consultation 2. Also, information regarding the potential of TAGs to investigate geographical origin is discussed in the following section.
6.5. Geographical origin of edible oils
6.5.1. Research relating to geographical origin of edible oils
Much work has been undertaken to differentiate edible oils according to their geographical origin, to determine Protected Geographical Indication (PGI) or Protected Designated Origin (PDO), rather than according to their botanical origin. While GO determination can be of great economic importance to exporting regions and nations, often due to perceived benefits of certain GO oils compared to others, geographical origin is a minor focus of this review which aims mostly to determine the adulteration of an oil with an oil from another botanical origin to support UK consumers. Determining the geographical origin of edible oils is covered in more detail in FSA-and Defra-funded project ‘Review of Capability of Methods for the Verification of Country of Origin for Food and Feed’ (FSA et al., 2024).
SIRA is a recognised method which is often beneficial in supporting the verification of origin of foods and several authors reported the application of SIRA to determine the provenance of edible oils (Banerjee et al., 2015); (Huang et al., 2017); (Bontempo et al., 2019); (Jeon et al., 2015). Banerjee et al. (2015) demonstrated that measurements of oxygen and hydrogen isotopes could identify geographical origin based on oxygen and hydrogen in meteoric water used for irrigating the crops for 43 commercial edible oils (21 extra virgin and 4 olive oils, 2 sesame oils, 2 sunflower oils, 2 palm oils, 2 walnut oils, 1 coconut oil, 1 pumpkin seed oil and 4 vegetable butters from USA, Europe and Africa). Bontempo et al. (2019) reached similar conclusions, aligning isotope ratios for EVOO from different cultivars, sampled during 2015-2016 from Argentina, Australia, France, Greece, Italy, Morocco, Peru, Portugal, Spain, Tunisia, Turkey, Uruguay and USA to verify origin down to country level to verify origin. Similarly, Jeon et al. (2015) successfully applied isotope ratio analysis of carbon, oxygen and hydrogen to differentiate sesame oils prepared from roasted Korean, Chinese, and Indian sesame oils.
Authors have also used carbon isotope ratios to identify authenticity issues when oils have been prepared from different groups of plants, namely when an oil from a C3 plant is substituted with that from a C4 plant (Banerjee et al., 2015); (Huang et al., 2017). Such an example is the substitution or dilution of an olive oil with corn oil. Banerjee et al achieved a limit of detection of 15%. Work by Huang et al., 2017 also reported success in differentiating oils of C3 origin (olive and soybean oils) from the C4 corn oil using carbon isotope ratio.
While SIRA alone can be applied to investigate the geographical origin of food, as highlighted in FSA- and Defra-funded project FS900435, many authors apply a combination of methods with the aim to build a more powerful tool. A study which included the study of the influence of ripening stage, cultivar and climate on quantifying isotopic ratio and fatty acid compositions (FAMEs) highlighted that the variability in δ13C and δ18O values of olive oil is determined by a complex combination of environmental, physiological and genetic factors (Portarena et al., 2015). Particularly, the results highlight the importance of the cultivar and ripening stage as determinants of isotope compositions in olive oil. The authors warn that these parameters must be taken into account in traceability studies.
Gumus and co-workers (Gumus et al., 2020) studied the geographical provenance of 49 Turkish olive oils from six different locations, measuring 61 different parameters including stable carbon isotope ratio, trace element composition, sterol composition, FAMEs and TAGs. In conclusion, the results obtained from this study confirm that geographic proximity and climate characteristics such as such as annual average temperature, rainfall amount, humidity amount and number of days covered with snow are effective in determining the geographical origins of olive oils. Trace element composition was influential in the differentiation of olive oils from Manisa regions while fatty acid composition and/or TAGs components were important in assigning provenance for oils produced in other regions of Turkey. The number of parameters included in this study highlights the complexities of differentiating PDO and PGI olive oils which originate from proximate regions of a single country. Lucic and co-workers (Lucic et al., 2023) also reported trace element analysis, in combination with stable isotope data (carbon isotope composition), as a promising tool to differential EVOO of Croatian origin.
Authors have combined SIRA analysis with 1H-NMR to determine the geographical origin of olive oils (Camin et al., 2016). The combination of these two techniques allowed the data from Italian olive oil and lower grade Tunisian olive oils to be split according to geographical origin and then the Tunisian data were further split into two groups, possibly relating to cultivar. The authors stated that, by combining isotopic composition with 1H-NMR data using a multivariate statistical approach, a statistical model able to discriminate olive oil from Italy and oil imported from Tunisia was obtained, with an optimal differentiation ability arriving at 98.5%. Interestingly, the parameters included in the model were not linked to the quality of olive oil and, therefore, the model is applicable for oils for which the quality is not comparable. The authors highlighted the need to further explore the applicability if the method by analysing oils sampled directly from the Tunisian cultivation sites and by including oils from a wider range of origins such as Spain. The combination of 1H NMR data with hydrogen and carbon isotope ratio data is also reported by (Alonso-Salces et al., 2015). The group applied the PLS-DA models to separate VOOs from Greece and detect non-Greek VOOs, achieving accuracy of over 93%.
In 2015, Kim and colleagues (Kim et al., 2015) combined IRMS (δ13C), 1H-NMR and FAMEs analysis to determine the authenticity of sesame oil. The reference dataset comprised 35 authentic oils prepared by the authors from 19 seed samples sourced from Korea and 19 samples of imported sesame seed. The seeds were roasted before oil extraction.
The important variables for assigning adulteration of sesame oil were the δ13C value from the stable isotope ratio data; three peak values from the 1H-NMR spectrum data and methylene presence between two carbon double bonds present in certain of the triacylglycerols present in the oils. The method was then challenged with 70 sesame oil samples and various combinations of oils. Application of IRMS, 1H-NMR and FAMEs analysis individually correctly identified that adulteration had or had not occurred (as applicable) in 18, 56 and 54 of the samples respectively. When combined, IRMS and 1H-NMR correctly assigned the authenticity status of 63 oils while IRMS plus FAMEs analysis correctly assigned 60 samples. When the data from all three technologies was applied, the authenticity of 65 of the 70 samples was correctly applied. Adulteration with corn oil was detected at ≥15 v/v %, soybean oils at ≥3 v/v % and sesame-flavoured oils at concentrations of ≥5 v/v %.
Regarding other technologies, (Horacek et al., 2015) applied genomic approaches to differentiate sesame seed DNA according to country of origin, aiming to identify variations in the genetic pool relating to geographical origin. The outputs were mixed. Regional geographic differentiation was successful where as long as seed and plant material is not transferred from one region to another, for example between regions of Uganda. However Kenyan samples clustered with samples from Turkey, Ethiopia and India since genetic material had been transferred to new regions during international breeding programs.
Pilot studies using Matrix-Assisted Laser Desorption/Ionisation-Time of Flight (MALDI-ToF) technology have reported assigned geographical origins to edible oils. Zeng et al. (Zeng et al., 2024) built a dataset of 40 samples. When OPLS-DA models were applied, the model was accurate in identifying the geographical origin of soybean oils from Argentina, USA, Brazil and Canada. Again, in pilot studies, Persuric et al. (Persuric et al., 2017) reported differentiation of cultivars which depicted different growing regions by studying the MALDI -ToF fingerprint of TAGs for Croatian EVOO, detecting adulteration of EVOO at the 1% level. Success has been reported (pilot studies only) in using MALDI-ToF with the further potential to determine structural identification if a MALDI-MS/MS platform was available. In a follow-up study which included some MS/MS in 2018 (Persuric et al., 2018), the group reported that MALDI-ToF MS analysis of TAGs was key in characterising the geographical (regional) origin of Croatian olive oils. The group compared TAG analysis by MALDI-ToF MS with GC-MS analysis of fatty acids and NIRS analysis, using PCA and PLS-DA. TAG profiling provided better classification ability than fatty acid analysis. NIR showed potential as a complementary rapid and non-targeted method for geographical (regional) origin of these Croatian EVOOs.
Again, in a small study, Kuo and co-workers (Kuo et al., 2019) were able to demonstrate that the MALDI-ToF profile of olive oil and rapeseed oil were sufficiently different to detect TAGs from both oil types, and dilution of olive oil with rapeseed oil was detected with a limit of detection in the range of 20-40%. The differentiation of other oils was not achieved in this particular study. MALDI offers a rapid test; once a sample has been mixed with MALDI matrix (5-dihydroxybenzoic acid) and sodium trifluoroacetate dissolved in tetrahydrofuran in a ratio of 2:1:1 (v/v/v), a drop is added to the MALDI plate to produce a ‘spot’ of sample. This is analysed in positive ion reflection mode. To form a single spectrum for each oil sample, six independent sub-spectra (500 shots per sub-spectrum) from different positions within a sample spot can be manually collected. Subsequently, spectra are baseline subtracted and analysed (Persuric et al., 2018). This process of analysis for a sample is easy, taking around 5 minutes to prepare the sample (or multiple samples) with no requirement for analyte purification or derivatisation and then less than 5 minutes to analyse a sample.
6.5.2. Conclusions relating to authenticity of geographical origin of edible oils
Stable isotope ratio analysis comprises a beneficial method to support the geographical origin authenticity testing of edible oils. This method relies on building a comprehensive dataset which underpins the technology comprising authentic samples of the geographical origin regions of interest. The outputs are only as robust as the underlying dataset. When SIRA is combined with other technologies, such as 1H-NMR and FAMES analysis, which also rely on robust datasets, greater certainty can be applied to the results.
Given the strong potential of TAG analysis in the authenticity of edible oil, it will be interesting to note the future research in analysis of TAGs by the rapid mass spectrometry platform of MALDI-ToF to investigate geographical origin, ideally involving larger studies than these pilot projects, incorporating larger numbers of samples and a wider breadth of botanical oil types. Advantages of MALDI ToF are that the analysis requires less than one microlitre of sample, thousands of samples can be analysed relatively quickly and these is assurance of no sample carry over from one analysis to the next, unlike in chromatography methods. Tests would first need to be completed to establish robust relative measurements and to prove organic matrix does not interfere with the low molecular weight molecules.
6.6. Genomics analysis for edible oil authenticity
This literature review details the many conventional edible oil authenticity tests using analytical chemistry-based methodology. However, these methods are not as successful in identifying the botanical or varietal composition of an edible oil or the admixture of an oil with a similar chemical composition. Deoxyribonucleic acid-(DNA-) based assays have proven to be useful in authenticating the species used in processed foods, as the residual DNA in a food sample directly represents the species/variety from which the food was produced. Therefore, once DNA has been successfully recovered from the food matrix, a wide variety of, mainly polymerase chain reaction (PCR)-based analyses, can be used to verify the botanical origin of the sample. Edible oils however, present unique challenges for analysis since DNA quality and quantity is easily compromised during oil production.
6.6.1. DNA quality and quantity in edible oils
Edible oils which have undergone minimal processing contain a relatively large concentration of intact DNA molecules when compared to processed edible oils since DNA degrades and is removed during processing (Corrado, 2016); (Raieta et al., 2015) (Xia et al., 2021). Xia et al. elegantly described the degradation of DNA during GM soybean oil processing. In that study, samples were taken during the industrial soybean oil extraction and refining process and then DNA recovered from those samples analysed to evaluate DNA quality and quantity. The results indicated that the DNA concentrations in soybean seeds, kernels, conditioned soybean, laminated soybean, extracted soybean, and meal (approximately 2358.500 µg/g) were significantly higher than those in crude oil (194.800 ng/g) and in subsequently refined oils (0.026–0.664 ng/g) (P < 0.05). It was found that organic solvent extraction and degumming were the two main steps in removing DNA and combined treatment with organic solvent and heating (110 °C) and heating at 240–250 °C were key steps in degrading DNA. Industrial processes such as these result in edible oils which contain comparatively low concentrations of small DNA molecules (Corrado, 2016). Robust and reproducible analysis of edible oils is constrained by the quality and quantity of DNA extracted from the oil.
6.6.2. DNA Extraction and purification
The recovery of DNA from edible oil is known to be problematic and a variety of different methods have been reported in the literature (Bojang et al., 2021; Contreras-Díaz et al., 2024; Raieta et al., 2015; Wu et al., 2023). Despite these reports, the best method for the recovery of PCR amplifiable DNA remains uncertain (Sebastiani & Busconi, 2017). Many of the published methods require a very large amount of starting material and one or more DNA precipitations to purify the DNA (Bojang et al., 2021; Contreras-Díaz et al., 2024; Raieta et al., 2015; Wu et al., 2023).
For the majority of edible oil samples, the availability of a large quantity of starting material is not usually an issue, however, methods which include the precipitation of DNA are less than optimal. Small fragments of DNA do not precipitate easily and can be lost in the supernatant when the DNA in pelleted by centrifugation. Some of the published methods used a relatively gentle precipitation condition, for example precipitation using cold isopropanol without incubation (Crawford et al., 2020), precipitation at 4oC for 2 hrs (A. O. Uncu et al., 2018), 30 mins at -80oC (Piarulli et al., 2019) or -20oC for 1hr (Alonso-Rebollo et al., 2017; Gomes et al., 2018; Ramos-Gómez et al., 2016). However, in recognition that more stringent precipitation conditions would be needed to recover as much DNA from the sample as possible, other studies precipitated the DNA overnight at -20oC (Contreras-Díaz et al., 2024; Gomes et al., 2018; Lucchetti et al., 2018; Raieta et al., 2015; Su et al., 2019; Wu et al., 2023).
More recently a study (Ramos-Gómez et al., 2023) avoided precipitation altogether when recovering DNA from olive, almond and hazelnut oils. Despite multiple studies being published by that group using DNA precipitation during DNA extraction from edible oils, the group appeared to be moving away from using precipitation as a way of purifying DNA. Unfortunately, the alternative methodology chosen included the use of phenol in a phenol:chloroform:isoamyl alcohol mixture. The use of phenol has been phased out in the majority of laboratory settings because of the need to handle it with extreme care due to its acute toxicity. Indeed, only a few of the studies found in this literature review used phenol at all (Alonso-Rebollo et al., 2017; Crawford et al., 2020; Raieta et al., 2015) reflecting the fact that it is a dangerous and unpopular chemical. DNA precipitation and the use of phenol cannot be recommended for the recovery of DNA from edible oils. The inclusion of DNA precipitation in so many studies and phenol in a handful is most probably due to the relatively low cost of the reagents used and the continued use of methods of handling DNA established 30 years ago in "Molecular Cloning" by Sambrook, Fritsch and Maniatis (1989), a laboratory manual which has served as the foundation of technical expertise in DNA laboratories, worldwide, for 30 years. Expertise in molecular biology has changed immeasurably in the last 30 years and the methods outlined in Molecular Cloning, (Sambrook et al., 1989), whilst fit for purpose at the time, are now outdated and do not take advantage of recent technological advances.
An alternative strategy for extracting DNA from edible oils is to use a commercial kit. Many analytical laboratories, Fera included, prefer to use commercial kits for DNA extraction because they offer a consistent and standardised approach using reagents which have been QC-checked and are certified for molecular biology procedures. Kit manufacturers use their huge resources to develop kits which are standardized for specific tasks and optimized for speed. Additionally, kits usually include DNA extraction by some form of solid phase, with a resin or a column, resulting in consistently good quality DNA from the majority of food matrices (H. Hird, personal observation).
For example, the Promega Wizard® Magnetic Purification System for Food kit uses a solid phase extraction system. In a study funded by the Food Standards Agency at Campden and Chorleywood Food Research Association (FSA Research and Development (R&D) Project Reference Q01095 (Dooley et al., 2006)) a comparison of two variations of DNA extraction from oil using this kit was made. The original protocol, developed in a previous study funded by the FSA (FSA R&D Project Reference Q01060 (Garrett et al., 2003)) using the kit on pelleted cellular material after oil had been centrifuged at 21,000g for 60 minutes was compared with the second protocol using the same kit, but without centrifugation and using the manufacturer’s recommended protocol. It was found that the original protocol, including the centrifugation step, recovered more amplifiable DNA from hazelnut oil. The study recommended that this protocol be incorporated into a Standard Operating Procedure (SOP) for the detection of Hazelnut oil, despite noting that the requirement for highspeed centrifugation would restrict the application of the SOP to a small number of enforcement laboratories (Dooley et al., 2006).
The Promega Wizard® Magnetic Purification System for Food kit was also successfully used by a group investigating olive oil cultivar origin and admixture in Turkish olive oils and the presence of plant oil adulteration in milk and dairy products (A. T. Uncu et al., 2015); (A. O. Uncu & Uncu, 2020).
However, a study published in 2018 which compared 5 different kits for the extraction of amplifiable DNA from cold pressed hazelnut oils and their centrifugal sediments, found that none of the kits assessed recovered amplifiable DNA immediately suitable for onward analysis (Lucchetti et al., 2018). The kits included in the study were: the Wizard Magnetic DNA purification System for Food (Promega, Milan Italy); the QIAmp DNA Stool Mini Kit (Qiagen, Milan Italy); the Nucleospin Food Kit (Macherey Nagel, Duren Germany); the GenElute Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, Milan Italy); and the Extract-N-Amp Plant PCR kit (Sigma -Aldrich. Milan Italy), all from very large and well-known multi-national suppliers of molecular biology reagents. It is surprising that none of the kits performed satisfactorily, even on the centrifugal sediments which would be expected to contain cellular debris and therefore a higher starting concentration of DNA-bearing material. Disappointingly, this study then suggested circumventing poor DNA recovery and quality by performing a total whole genome pre-amplification step prior to onward analysis. The use of a pre-amplification step on DNA which is low quality and fragmented cannot be recommended since it can introduce bias, artifacts and loss of genetic information for downstream analysis.
From the literature, the 2 most successful commercial kits available appear to be the Nucleospin Plant II kit (Macherey-Nagel, Düren, Germany) (Amaral et al., 2022) (Pasqualone et al., 2016; Xia et al., 2021) and the Norgen kit for olive oil (Norgen Biotek, Thorold, Canada) (Chedid et al., 2020; Christopoulou, Figgou, et al., 2024).
The Nucleospin Plant II kit has been updated and now features improved buffer composition for a higher yield and purity and an optimised silica membrane in the form of a spin column with an improved DNA binding capacity. The kit can extract the DNA from up to 5ml in approximately 30 minutes.
Authors (Amaral et al., 2022) used the Nucleospin Plant II kit to successfully recover amplifiable DNA from argan, olive and soy oil mixtures, although the oils had been centrifuged at 18,514g prior to DNA recovery from the resultant pellet. In a separate study DNA was successfully recovered from olive oil centrifuged at low speed (3500 rpm for 10 minutes) prior to recovery of DNA using the kit from the cellular debris (Pasqualone et al., 2016). Other studies successfully recovered DNA from olive oil samples using the kit without prior centrifugation (Kyriakopoulou & Kalogianni, 2020); (Xia et al., 2021). The naming of the kit in these studies is generic and it is not clearly outlined which version of the kit was used, however the kit was updated in 2014 and the original version is no longer available and so it is most probable that, at least for the more recent publications, the Nucleospin Plant II kit was used.
A relatively new kit on the market, the Norgen kit for olive oil, is similar to the Nucleospin Plant II kit as it also includes a spin column. This kit recovers DNA from a relatively small sample size (0.5 mL) using a combination of heat treatment, homogenization and a proprietary lysis buffer. PCR inhibitors are effectively removed during the washing steps and the purified genomic DNA is ready to use in downstream applications, for example PCR, in less than 30 minutes (Olive Oil DNA Isolation Kit (accessed 16/12/2024)).
Chedid et al. (2020) compared an in-house buffer-based protocol with the Norgen Olive Oil Kit and concluded that the Norgen Olive Oil Kit required less time compared to the buffer-based protocol and was considered more reliable in providing olive oil DNA isolates which were more consistent in subsequent down-stream analyses. In a further two studies a Greek group, developing sensors for molecular traceability of olive oil, used the Norgen Olive Oil kit to successfully recover amplifiable DNA from soya, sunflower, sesame, corn, hazelnut, almond and olive oils (Christopoulou, Figgou, et al., 2024 & Christopoulou, Mamoulaki, et al., 2024).
6.6.3. Summary for DNA recovery from edible oils
The recovery of amplifiable DNA from edible oils is not a trivial task and there is currently no consensus as to the best method or protocol to use. Two commercial kits, the Nucleospin Plant II kit and the newer Norgen Olive Oil kit appear to be becoming popular in the literature and seem to be successful in recovering DNA from edible oils. However, it is difficult to compare across studies as the quantity and quality of DNA in edible oils varies so much and no two studies use the same starting material. The most suitable method for the recovery of PCR-amplifiable DNA from edible oils remains uncertain.
6.6.4. The use of molecular markers in edible oil traceability and authenticity
A molecular marker is a region of DNA associated with a specific location in the genome showing polymorphism of the nucleotide sequence in different individuals, due to insertion, deletion, point mutations, duplication or translocation. Different types of molecular markers have been developed for a range of applications (Sion et al., 2021) and in the authenticity and traceability of edible oils no single technology has emerged as the most suitable. Many technologies are used in tandem, one of the most powerful being High Resolution Melting (HRM) analysis.
6.6.5. High Resolution Melting Analysis
HRM analysis is a powerful molecular technique which is a reliable and reproducible method of analysing PCR amplicons. HRM detects small variations between sequences based on differences in their melting temperature. During HRM analysis PCR is performed in the presence of a dye that binds to double-stranded DNA (dsDNA). This dye is highly fluorescent when it is intercalated to dsDNA but shows low levels of fluorescence when unbounded. After the PCR is completed, amplicons are denatured to single stranded DNA by slowly increasing the temperature. The florescent dye is released as the dsDNA is denatured causing a reduction in the emitted fluorescence. A melting curve can be constructed from the diminishing fluorescence emission values plotted against the increasing temperature. The shape of this curve will depend on the amplicon length, guanine-cytosine content, melting temperature and nucleotide composition. Samples can be distinguished according to their unique melting curve (Pereira et al., 2018). HRM is not a stand-alone technique since it is performed after PCR. It can though be coupled to virtually any PCR-based analysis as a final confirmatory step, without affecting the initial PCR amplification reaction. However, PCR reaction success is critical to achieve specific amplification of the intended targets for HRM to be accurate (Pereira et al., 2018).
6.6.6. Short tandem repeats
While a lesser focus of this review compared to identifying methods to differentiate oil species, researchers have had some success in differentiating different OO and EVOO cultivars. Differentiation of these cultivars often relates to verifying geographical origin of PDO olive oils. This relates to short tandem repeat analysis and work. Short tandem repeats (SSR) are simple sequence repeats where there are multiple repetitions of short motifs (2-6 base pairs) of sequence, which are flanked by conserved sequences. Because of the repetitive nature of the sequence, it is likely that during DNA replication units of the SSR are erroneously added or removed creating regions of the genome with different repeat numbers between individuals/varieties (Lanubile et al., 2024). Additionally, SSR have a high polymorphism level and are widely distributed along the genome (Muzzalupo et al., 2015). SSR are easily analysed by PCR-based techniques as the conserved regions flanking the repeated sequences can be used to locate primers, facilitating amplification across the SSR. During PCR an SSR usually gives a single product which relates to the number of repeats at that locus. Some SSR are highly informative and different cultivars have different numbers of repeats at that locus, resulting in PCR products of easily distinguishable sizes, whereas other loci are not informative and all cultivars will have the same number of repeats resulting in identically sized PCR products. A panel of SSR is usually analysed to develop a pattern used as a key for the cultivar/variety of interest.
Research has been completed in this area (Montemurro et al., 2015; (Muzzalupo et al., 2015), Contreras-Díaz et al., 2024; Gomes et al., 2018; Raieta et al., 2015). These studies were able to show discrimination between olive cultivars and their products between a relatively small number of samples in a very small geographical range. These studies did not include samples from a wider range of geographical origins and cultivar type. Until these SSR panels are subjected to a wider variety of samples it is not known whether the discrimination observed in the studies would remain across a wider sample range. This problem is exacerbated as the studies did not use the same SSR markers, with only a few being used in more than one study, limiting the comparison of methodology and sensitivity. Databases containing a consensus list of alleles for a validated standard set of SSR markers with high power of discrimination, reproducibility (low peak stuttering), strong peak signal and absence of null alleles, are available for olive genotyping (Doveri et al., 2008); (Baldoni et al., 2009) and have led to the implementation of public SSR-marker databases, for example the Italian “OLEA db” (Bartoleni, 2008), the Olive Genetic Diversity Database (OGDD) relative to the Mediterranean germplasm (Ben Ayed et al., 2016), the Mendoza Argentina database (Torres et al., 2014) and the Algerian National Olive Germplasm Repository (ITAFV) (Haddad et al., 2020). The use of these databases and using SSR markers across studies should lead to methods with a much wider applicability.
SSR analysis can be performed on any plant species DNA and in 2017, a study was published on an SSR-based method for hazelnut oil analysis (Lucchetti et al., 2018). A panel of 9 SSR were used to begin to develop a traceability system for cold pressed hazelnut oil, however the analysis system was poor. The DNA extraction methodology so inadequate that virtually no DNA was extracted from the samples of 100% hazelnut oil and a whole genome pre-amplification step was needed, which is not recommended. The SSR markers were developed solely for the hazelnut genome, and so methodology might be used to detect OO adulterated with hazelnut oil in the future (Lucchetti et al., 2018).
6.6.7. Single nucleotide polymorphisms
Single nucleotide polymorphisms are point mutations causing a variation in DNA sequence involving a single nucleotide. The use of SNPs is more common for animal-derived products compared to plant derived products, nevertheless SNPs have been used in edible oil authenticity and traceability (Lanubile et al., 2024).
The advantages to SNPs are thus: a) they are the most abundant genetic markers; b) they have lower mutation rates and are stably inherited; c) they can be detected within a low size range of amplicons (e.g. 100 base pairs) so they are a better choice for DNA that has been degraded and extracted from a complex matrix such as olive oil, d) they are mostly biallelic (a specific locus in a genome contains two observed alleles), e) they are able to differentiate very similar cultivars that may differ in only one nucleotide in a specific locus (Batrinou et al., 2020).
Although large panels of SNPs have been developed for analysing olive germplasm resources (Sebastiani & Busconi, 2017), there are only a few studies on the use of SSR for authenticity and traceability of edible oils in the literature.
Chedid et al. (2020) showed that SNPs had a higher discriminatory capacity compared to SSR markers. Additionally, they found that although SSR-HRM was more efficient in distinguishing monovarietal olive oils, SNP-HRM was better at discriminating olive oil blends.
Piarulli and co-workers (Piarulli et al., 2019) investigated only 3 SNPs in a study on Italian EVOO samples, and found that discrimination between different Italian olive cultivars was poor.
In a rather convoluted study by Uncu et al. (A. T. Uncu et al., 2015), SNP analysis was combined with amplicon digestion to develop assays for the detection of admixture in Turkish OO. This methodology cannot be recommended as anything other than a research tool and will not be discussed further.
6.6.8. Real-Time PCR in OO authenticity
Real-time PCR (qPCR) is more sensitive than conventional PCR and enables the detection and quantification of DNA in very low amounts. Additionally, the molecular markers used in qPCR are designed to amplify small fragments of DNA reducing the problems resulting from DNA degradation.
An interesting study by Ramos-Gomez and co-workers (Ramos-Gómez et al., 2016) showed that it was possible for the detection of olive in oils and derived foods based on the relative quantification of olive DNA. The system was able to detect amplification of DNA as low as 0.1% of olive content and olives as ingredient in 12 commercial food products. Although quantification of the olive content was not possible.
Successful quantification has however, been claimed in a study (Amaral et al., 2022). In this study novel qPCR assays were developed specific for olive, soybean and argan, as an approach to detect olive and soybean DNA in argan oil. The system was able to detect argan, olive and soybean DNA down to 0.01 pg, 0.1 pg and 3.2 pg, respectively. The study outlined an approach to quantify the amount of olive or soybean oil in argan oil, however, the results were based on a very small sample set and would need to be verified on a wider variety and greater number of samples.
A qPCR approach was used to differentiate Olea europaea var Sylvestris (wild-type olive) from Olea europaea L. var Europaea (cultivated olive). The oil of the wild-type of olive tree, the ancestor of the cultivated olive, is considered to be more nutritious with increased antioxidant activity compared to the common cultivated type. This has led to the wild-type of olive oil having a much higher financial value. As cultivated and wild-type olives have similar phenotypes, an analytical method was developed to distinguish Olea europaea var Sylvestris (wild-type olive) from Olea europaea L. var Europaea (cultivated olive). The method was based on allele-specific qPCR, using a SNP present in the two olives’ chloroplastic genomes. The method was able to detect 1% content of the wild-type olive in binary DNA mixtures of the two olive species. This work would need to be extended to verify that DNA from both tree types could be identified in mixtures of oil, however, the approach shows promise.
In a similar approach qPCR systems for hazelnut and almond were developed for their identification in olive oil. The results were promising in that the study demonstrated that the methodology detected olive oil adulteration with up to 5% of hazelnut or almond oil. The study also used a nested-qPCR assay, which increased the sensitivity 2-fold, however as with whole genome pre-amplification a nested PCR approach is not recommended (Ramos-Gómez et al., 2023).
6.6.9. Barcoding for OO authenticity
For many applications, PCR primers are usually designed to amplify a single and well-known target sequence and can be considered species-specific. DNA barcoding involves amplifying regions that are common in different taxonomic groups but sufficiently polymorphic to identify or distinguish different species DNA, essentially a method of taxonomic identification based on the amplification and sequencing of predefined genomic regions. For plant species, there is no consensus on the best sequences to be used, but sequences such as trnL, rbcL, matK, the spacer trnHepsbA, the second internal transcribed spacer (ITS2) of nuclear ribosomal DNA and their combination, usually provide the ability to uniquely identify a plant species (Corrado, 2016).
A small number of studies have used barcoding for OO verification and traceability. A small 2015 study (A. T. Uncu et al., 2015) used the differences in the length of the trnL sequence to authenticate the botanical origin of olive oil and to identify small quantities of seed oil in OO.
The trnL sequence was also used in a study to develop and optimise a PCR based system specific for olive identification in edible oils (Alonso-Rebollo et al., 2017), which did not cross react with the DNA from other oleaginous species (canola, soybean, sunflower, maize, peanut and coconut).
6.6.10. DNA sensors for olive oil authenticity
In a departure from the usual methods of SNP analysis, a Greek group have been developing DNA sensors which can be read by eye. This group developed two sensors based on the same technology: a multi-allelic DNA sensor for OO cultivar verification and a multi-species DNA sensor for the identification of non-olive plant DNA in OO (Christopoulou, Figgou, et al., 2024; Christopoulou, Mamoulaki, et al., 2024).
The multi-allelic sensor for cultivar verification required PCR amplification, prior to a DNA polymerase-driven allele discrimination reaction. The products of these reactions were then applied to the device where the fragments were captured via hybridization and visualized by antibody-functionalized gold nanoparticles generating an 8-spot colour string for 8 alleles. The accuracy of the method was evaluated with 7 olive cultivars and the results were in full concordance with sequencing data (Christopoulou, Figgou, et al., 2024).
Of potentially more interest for OO authenticity is the second DNA sensor. This multi-species DNA sensing device was developed to identify the presence of olive and 6 other plant species: corn, sesame, soy, sunflower, almond, and hazelnut. The methodology was similar to the multi-allelic sensor in that following a single PCR reaction, with one pair of common primers for all 7 plant species, a 20 min multiplex plant-discrimination reaction by DNA polymerase was performed using a mixture of seven plant-specific primers. As before, the products of these reactions were then applied to the device where the fragments were captured via hybridization and visualized by antibody-functionalized gold nanoparticles as reporters. Spatially distinct regions containing immobilized capture oligonucleotides as molecular recognition elements were established for each plant species. Products corresponding to the plant species are visualized in the form of red spots in the appropriate regions of the sensor. In the absence of adulteration, only two spots appeared on the sensor: the control spot and the olive spot. The appearance of a spot in a different position from the olive indicated the presence of another vegetable oil in the sample with the position of the spot indicating the identity of the plant species (Christopoulou, Mamoulaki, et al., 2024).
6.6.11. Conclusion to genomics applications in relation to edible oil authenticity
The analysis of DNA from edible oil is not straightforward. The greatest impediment to reliable and reproducible analytical results is the recovery of DNA from the oil matrix. Cold pressed oils contain a relatively large amount of good quality DNA whereas processed oils contain very little DNA which is also degraded. This presents a problem in itself. For example, refined oil in a cold pressed oil would be difficult to discern as the DNA portion derived from the refined oil would be much lower in comparison to the DNA portion from the cold pressed oil.
Some of the analytical techniques outlined above show promise in being able to detect adulteration and substitution in a small subset of edible oils. However, with the exception of the CCRFA method for hazelnut DNA, none have been taken anywhere near validation and none are ready for implementation.
6.7. Other techniques for oil authentication
Other techniques which are less represented in the literature were considered for applicability to authenticate edible oils by evaluating the recent published data produced using technologies which are not otherwise covered in this review. The aim here was to identify any additional promising emerging techniques in addition to those detailed above. With the exception of the use of sensors and e-nose, other techniques used to determine edible oil authenticity show little consensus and are based on single publications (Table 4). A deeper consideration of e-nose technology may be of benefit in the future, but the quality of the papers referenced below does not provide a convincing argument that the other technologies should be widely used for the detection of adulterated oils.
For e-nose, the number of papers captured during the literature search compared to those for other emerging technologies may be skewed since ‘e-nose’ was used as a search term. However, it must also be considered that the term ‘e-tongue’ was also included and only one paper was captured for this technology. The concept of using sensors to detect adulteration tends to lack the supporting data that is provided by analytical measurements and may be better suited to screening in a field environment than implementation in a well-equipped laboratory.
7. HorizonScan™ historical data to inform on edible oil authenticity risks in the global supply chain
The HorizonScan™ tool (Fera Science Ltd, UK) is a subscription-based service which monitors global food integrity issues. While RASFF logs data for Europe, HorizonScan™ collects data on a daily basis from across the globe, comprising all data for food issues logged by official routes over the last twenty-two years. Subscribers can access issues emerging in the past 14-28 days. The tool can therefore be used to provide a rapid overview of current food issues. This project however has accessed historical issues reported over the past ten years. These data are of benefit since we often observe repeats in cycles of past authenticity issues and thus can inform and plan for future issues by understanding historical incidents for edible oils. The tool was accessed for this project to inform regarding the scale and scope of breaches in edible oil authenticity claims over the last ten years. The data focus on the edible oils in scope but, as a minor component, will also provide a broader scene relating to issues reported for all edible oils.
HorizonScan™ data were interrogated for the 10-year period between 1st October 2014 and 30th September 2024. First of all, the number of global reports per commodity were recorded in a pie chart (Figure 1), with HorizonScan™ data searched for authenticity incidents relating to fish oil, milk fat, olive oil (excluding infused oils), other vegetable and fruit oils, fats and infused oil, palm oil, pepper oil, rapeseed (canola) oil, soybean oil and sunflower oil. Authenticity events searched for adulteration/substitution, expiry date changes, fraudulent health certificate/ documentation, unapproved premises, produced without inspection and unauthorised/unsuitable transport and all events fell into the adulteration/substitution category. The number of incidents reported across time is plotted in Figure 2. Finally, the numbers of incidents reported according to exporting nation are plotted in Figure 3. A number of these incidents are of unknown origin, meaning that the reporting source did not provide details of the exporting country, so no further information is available. The proportion of mineral oil incidents per edible oil type are detailed in Figure 4 for the same timeframe.
All issues relating to palm oil related to the presence of illegal Sudan dyes in the oil. Palm oil is the main cooking oil used in Ghana, which is reflected in Figure 3. Of the issues relating to olive oil, 30% related to presence of other oils and 61% related to a poorer quality oil being labelled as EVOO. Of the issues relating to ‘other vegetable oils’, 42% of these related to mustard oils and 15% to coconut oils being incorrectly represented (were not authentic). Many of the issues relating to olive oil involved the incorrect assignment of extra virgin olive oil, being replaced by refined olive oil or other vegetable oils. Gutter oil and oil prepared from animal waste was also represented in 8% of cases).
The addition of mineral oil to edible oil in the supply chain is clearly a cause for current concern. Incidents of mineral oil presence have been logged since 2019 and have risen steadily over many of the intervening years to 2024. This poses a serious safety issue in addition to contravening authenticity requirements.
The data above provide a rapid overview of recent food issues. An understanding of these historical issues in edible oil supply will allow us to plan for future areas for focus for previously reported incidents for edible oils. In addition to these data, the continuous scanning of current changes in the food supply chain, such as poor harvests or disruption of supply due to geopolitical events, is important to support the prediction of areas vulnerable to new types of fraud.
8. Access to Fapas® Proficiency Testing oil authenticity data
Written by Mark Sykes, Scientific Advisor to Fapas®
8.1. Introduction
Olive oil authenticity proficiency tests (PTs) have been a recent development for Fapas®, only introduced following the 2021 closure of the EU Horizon project OLEUM developing methods of analysis for olive oil authenticity. Prior to the OLEUM project, there had been occasional enquiries for PTs for olive oil authenticity but it was hoped that OLEUM would drive sufficient interest to make PTs viable. Fera was a partner (and work package lead) in the OLEUM project, and Fapas® was specifically tasked with providing the various blends of oils for the method validation studies.
Two types of olive oil PT were introduced by Fapas® for authenticity testing purposes. The fatty acid profile PTs built on existing fatty acid profile PTs in other matrices which had previously been more limited in the scope of fatty acids to be reported. A specific authenticity PT for olive oil was introduced which was designed to be method-independent, to detect deliberate adulteration of olive oil samples. The outcomes of the few PTs run so far are described in this section of the report.
In the fatty acid profile PTs, fewer than half the participants were from European countries, despite the OLEUM project being a European-wide consortium project (including UK and Turkey).
8.2. Olive Oil fatty acid profile PTs
Two PTs have run so far (a third is currently in progress at the time of writing, January 2025).
8.2.1. Fapas® PT 14263
This PT was run over the period December 2022 - January 2023 and was advertised in the Fapas® programme from its launch in September 2021. The PT attracted 15 laboratory registrations, of which 12 submitted results by the deadline.
The test material was a commercially-available extra virgin olive oil (EVOO) with antioxidant butylated hydroxytoluene (BHT) added at 0.01%. Sample bottles (30 ml aliquot of oil) were nitrogen sparged of the headspace.
Participants were instructed to report a defined selection of 12 fatty acids, in units of g/100g of sample. The final list of fatty acids was decided following homogeneity analysis and general fatty acid profiling by the subcontracted laboratory.
As with the majority of Fapas® Food Chemistry PTs, the analyte assigned values were calculated as the consensus of participants’ results, according to the Fapas® Protocol Part 1. The PTs were run within scope of accreditation to ISO 17043. The standard deviation for proficiency assessment (SDPA or σp) was derived for each fatty acid from fitness-for-purpose experience and expert advice on historical PTs, largely dependent on concentration. Performance assessments were provided as z-scores. This was a consistent approach for fatty acid PTs.
In PT 14263, the assigned values ranged 0.0613 – 11.5 g/100g sample, except Oleic acid at 69.4 g/100g sample. The percentage of z-scores within ±2 (informally, the satisfactory range) ranged 50-92%, mostly 80-90%. Lignoceric acid (C24:0) had the 50% z-scores within ±2 and was bimodal in distribution but also at the lowest concentration of 0.0613 g/100g sample. Table 5 provides the summary data for the PT.
Oleic acid performance assessments were issued for information only due to the high uncertainty of the assigned value. This is despite oleic acid being the most abundant fatty acid present in olive oil by some considerable margin. The ratio of uncertainty (u) to σp was 0.44, which exceeds the critical value of 0.35 (Fapas® Protocol Part 1) for the issuing of fully evaluative performance assessments. The distribution of oleic acid results was not normal but there is no rationale for this observation. Figure 6 shows the distribution of oleic acid results as a kernel density plot.
Participants could optionally report method information. Ten of the 12 active participants reported some method information, of which a summary is: <1g analytical subsample, no internal standard used, no separate fat extraction, methylation to ester, determination by GC-FID. One laboratory reported the use of 1H-NMR.
8.2.2. Fapas® PT 14286
This PT was run over the period December 2023 - January 2024 and was advertised in the Fapas® programme from its launch in September 2022. The PT attracted 15 laboratory registrations, of which all 15 submitted results by the deadline.
The test material was a blind re-use of that used for PT 14263 (following storage at -20 °C). Only three participants were the same in both PTs 14263 and 14286. The PT was otherwise run in the same way as 14263.
The consensus assigned values ranged 0.0600 – 11.2 g/100g sample, except Oleic acid at 69.5 g/100g sample. The percentage of z-scores within ±2 (informally, the satisfactory range) ranged 73-100%, mostly 80-100%. Table 5 provides the summary data for the PT. Although the test materials were re-used from the previous PT, the statistical analysis treated the data independently.
Oleic acid this time was issued fully, although still not normally distributed, but alpha-linolenic acid (ALA, C18:3 n-3) was issued as info only (u/σp = 0.41). Figure 7 shows the distribution of oleic acid results as a kernel density plot. The bimodality of lignoceric acid (C24:0) observed in 14263 was not seen this time to the same extent and its assigned value was almost identical.
Method information was provided by 14 of the 15 participants. The general summary of the analytical methods was the same as in 14263, except no laboratory reported using a determination method other than GC-FID.
A possible reason for the kernel density observed is as follows: the fatty acid profile analysis corresponds to a single method. However, Oleic acid (C18:1 n-9 cis) co-elutes with a minor fatty acid that might be present in some oils, cis-Vaccenic acid (C18:1 n-7 cis). Participants using a high-resolution GC column will separate these two fatty acids but otherwise partial or complete co-elution is likely to occur. In PTs involving a test material comprising 100% olive oil, the cis-Vaccenic acid (C18:1 n-7 cis) could be a negligible component but in other vegetable oils, it might be more prevalent (maybe 5% the abundance of Oleic acid (C18:1 n-9 cis)). While this does not quite account for the shoulder on the kernel density plot, this could be a contributing factor. In some of our vegetable oil fatty acid profile PTs, Fapas® requests an analyte that is the sum of Oleic acid (C18:1 n-9 cis) and cis-Vaccenic acid (C18:1 n-7 cis), in acknowledgement that some labs cannot chromatographically resolve these and simply report the combined peak. However, this is not the case for olive oil PTs.
8.2.3. Olive Oil adulteration PTs
These PTs were marketed as olive oil authenticity PTs and sat within the oils and fats series of the Fapas® programme (rather than the authenticity series which historically had comprised meat and fish species authenticity tests). Specific marketing was undertaken for these PTs to make sure participants were aware of their existence. Since no very similar PTs existed within the Fapas® schedule of accreditation, these were not run with any claim to accreditation to ISO 17043, although they did follow the same processes.
For each PT, three sample types were prepared, blind labelled as Test Material A (TMA), Test Material B (TMB) and Test Material C (TMC). One of the test materials was always 100% EVOO, the other two were adulterated with other oils at levels which would be economically advantageous. The EVOO was sourced from a reputable supplier in Italy, on recommendation by the Fapas® agent in Italy. The adulterant oils were sourced from reputable UK suppliers but it was less important for these to have the same level of traceability and authenticity as the EVOO. The preparation of the test samples was a witnessed activity, which replaced the need to undertake analytical homogeneity testing, the capability of which was itself in doubt for this type of PT. Participants were simply instructed to report whether each sample was adulterated or not, with vegetable oils at adulteration levels between 5 and 100%. Participants had the option to identify what the adulterant was and if there was any other oil contamination (i.e. not at economically viable adulteration levels).
8.2.4. Fapas® PT 14244
This PT was run over the period March-April 2022 and was advertised in the Fapas® programme from its launch in September 2020. The PT attracted only 4 laboratory registrations, of which 2 submitted results by the deadline.
TMA was EVOO + 30% hazelnut oil (1 participant correctly identified adulteration but identified sunflower high oleic oil as the adulterant), TMB was EVOO + 15% avocado oil (no laboratories identified adulteration), TMC was 100% EVOO (both laboratories correctly identified no adulteration). No useful method information was reported by either participant.
8.2.5. Fapas® PT 14264
This PT was run over the period December 2022-January 2023 and was advertised in the Fapas® programme from its launch in September 2021. The PT again attracted only 4 laboratory registrations, of which 3 submitted results by the deadline.
TMA was EVOO + 25% walnut oil, TMB was 100% EVOO, TMC was EVOO + 25% soya oil. All three laboratories correctly identified which samples were adulterated, however, one laboratory identified the adulterant as soybean in TMA and TMC.
No method information was reported by any participant.
8.2.6. Fapas® PT 14287
This PT was run over the period December 2023-January 2024 and was advertised in the Fapas® programme from its launch in September 2022. The PT attracted only 3 laboratory registrations, of which all 3 submitted results by the deadline.
TMA was EVOO + 25% soya oil, TMB was EVOO + 25% walnut oil, TMC was 100% EVOO, i.e. re-use of the test materials from PT 14264 but re-labelled in a different order. All three laboratories correctly identified which samples were adulterated, however, one laboratory identified the adulterant as rapeseed in TMA and TMB. This was not the same laboratory that identified soybean as the adulterant in both adulterated samples in PT 14264.
One laboratory reported method information: determination of fatty acid profile. This was not the same laboratory that identified rapeseed as the adulterant oil.
8.3. Discussion of proficiency testing for edible oils
None of the olive oil PTs attracted the level of participation that was anticipated by the running of the OLEUM project. Fapas® has a customer base of several thousand laboratories world-wide and established PTs for fatty acids analysis attract 40-90 participants. Although it’s normal for a new PT to attract only a few participants initially, the fatty acid profile PT has a long-established method and is not very different from other fatty acid profile PTs (other vegetable oils, high fat finished products, for example). The 15 participants attracted to these PTs were only just sufficient for the statistical analysis to be viable. The same PT currently in progress (January 2025) has attracted 18 registrations.
The olive oil authenticity PTs were particularly disappointing in attracting so few participants. These were not quantitative PTs so the statistical limitation of number of data points on issuing assessments didn’t apply in the same way. Hence, these PTs were run with an expectation that interest would grow once it was established. Having now run three such PTs with no improvement in interest, these PTs have subsequently been discontinued in the Fapas® programme. In context, meat and fish authenticity PTs typically attract about 40 participants.
The fatty acid profile PTs, although not explicitly designed for the detection of adulteration, would represent the most common method of determining the type of oil by most laboratories that routinely undertake analysis of fats and oils (for any purpose). In fact, one participant in one of the authenticity PTs did use fatty acid profile as their method. Therefore, it seems logical that fatty acid profile would be the initial or primary method of detection of adulteration. The principal fatty acid in olive oil, oleic acid, had non-normal distributions in both PTs, and the assigned value and performance assessments were issued for information only in the first PT. Oleic acid in the second PT was issued as fully evaluative, although the uncertainty of the assigned value was borderline for this decision. This reduces the confidence in capabilities of laboratories to reproducibly provide a reliable fatty acid profile which might be used to detect adulteration of EVOO.
The olive oil authenticity PTs were disappointing in their outcomes, not just the low participation. Failure to detect adulteration in the first PT and misidentification of adulterant oils is not confidence-inspiring. The adulterant oils were chosen based on those used in the OLEUM project, so there was nothing unusual in the way the test materials were prepared.
EVOO is a high-priced commodity which can only be produced in certain climates so having the ability to accurately check its authenticity should be considered a primary consideration It is conjectured that one possible reason for the relative low interest and poor outcome of these PTs is a lack of engagement of the olive oil industry relative to the scale and likelihood of the adulteration problem. This is very subjective, however, a similar situation applies to the herbs and spices authenticity PTs run by Fapas®. These PTs are just about viable to run with a few more participants than seen in the olive oil authenticity PTs but not to the same extent as the meat and fish authenticity PTs. The meat and fish PTs have been established for many years and there are religious, cultural, animal welfare and consumer expectation reasons why the authenticity of meat and fish is much more prevalent in the food testing industry. Olive oil has not been subject to the same media scrutiny as meat adulteration or, to a lesser extent, herbs and spices.
For edible oils, other than the above rounds managed by Fapas®, no other proficiency testing rounds provided by alternative PT providers were identified, which further highlights the lack of international appetite for such proficiency testing.
9. Databases and quality control used in edible oil testing
9.1. Databases
The EU Horizon 2020 project aimed to identify an inventory of the databases for olive germ plasms and olive oils and their chemical and organoleptic characteristics. Sixteen databases were identified (OLEUM Project, Deliverable 5.1), of which 10 are publicly accessible. The accessible databases identified are reproduced in Table 6 as taken from the project’s reports. The report highlighted that an accessible database does not necessarily mean that the available information can be partially or entirely re-used in another database and it is essential to contact each curator to verify the applicability of the data.
As mentioned by a stakeholder in Appendix 1, the McCance and Widdowson’s ‘composition of foods integrated dataset’ is accessed by certain testing labs, focussing on nutrient content of foods in UK supply but may be out of date regarding, for example, the latest cultivars of plants.
Databases are also held by commercial companies which offer testing services in edible oil authenticity. These are detailed on the Food Authenticity Network website and are detailed below in Table 7.
9.2. Quality control in edible oil testing
Two key aspects in quality control of analytical testing methods are the use of reference materials to which to standardise testing data and the outcomes of proficiency testing of the testing providers. These aspects, in relation to edible oil testing, will be discussed below.
9.2.1. Reference materials for edible oils
Few reference materials are available and this is a limitation in edible oil testing. As discussed previously (FSA et al., 2023, 2024), preparation of reference materials requires much time and financial cost. To provide reference materials to determine adulteration of oils, this requires to first source (trusted sourcing parties in the supply chain needed) and verify (inter-laboratory testing incurs financial cost) authentic materials and then to prepare mixtures of these materials at known concentration, verifying by testing that they are homogenous. The overseeing by trusted individuals of the preparation of seed oils by small producers who use micro oil presses may be a beneficial way to source authentic oils for use as reference materials.
As reported in the EU Horizon 2020 project, for sensory analysis of VOOs, at present only reference materials from natural matrices (authentic olive oils that are used as reference samples of sensory defects provided by IOC, samples from proficiency tests, or certified by at least three accredited sensory panels) are available for training of sensory panels; they are effective in resembling real samples, although they have some limitations in their use over time (e.g. limited availability, difficult to obtain, low homogeneity year by year). Two reference materials were prepared but these related to quality (oil degradation) rather than to authenticity and their future applicability and/or availability is not clear from the project report.
Reference materials relating to olive oils discussed in Section 10 on Fapas® proficiency testing are available from Fapas®, comprising EVOO for fatty acid profiling proficiency testing. For olive oil proficiency testing, 100% EVOO is available or EVOO containing 30% hazelnut oil, EVOO containing 15% avocado oil, EVOO containing 25% walnut oil and EVOO containing 25% soya oil.
Reference materials also exist for certain analytes, such as fatty acids. However, these are not in an oil matrix. While they can be used to determine the level of the analyte, this does not necessarily equate to the level of each oil type in a sample. The only reference materials identified during the literature review and stakeholder engagement were those prepared by Fapas® as detailed above. No Certified Reference Materials for edible oil authenticity were identified. Stakeholders highlighted the critical lack of reference materials as a severe limitation to the authenticity testing of edible oils (Appendix 1).
10. Conclusions to the literature and data review
As demonstrated in this literature review, while much research has been conducted to investigate the applicability of various approaches to the authenticity of edible oils, apart from for olive oils, harmonisation of methods is lacking for edible oils. While many studies have been conducted, many are severely limited in the low number of samples and the narrow range of sample types analysed. Table 8 summarises the suitability of the various methods which show potential for the authenticity testing of edible oils and provides an approximate instrument time required to perform sample analysis on the technologies which show most potential.
The direct detection of adulteration has been discussed above using one of several fast techniques such as spectroscopy techniques without performing any separation or extraction steps. However, while these methods can be rapid, such methods can suffer inaccuracy and low sensitivity for the detection of adulteration. The methods often require to be underpinned by large datasets (Green & Wang, 2023) which is a limiting factor in the widespread application or rapid deployment of the methods. In particular, the robustness of these methods might be limited by the fact that only a small number of specific variables can be used for statistical analyses to classify edible oils or animal fats, whereas in chromatographic separation approaches, many FAs and TAGs compounds, including minor components in most cases, can be used as variables for statistical analyses. The detection of adulteration can be further improved by applying a chromatographic approach combined with mass spectrometry (such as HPLC-MS) since the efficient separation by chromatography and exact identification by mass spectrometry enables more comprehensive analysis, in the sense that it enables the determination of intact TAGs, allowing for the evaluation of potential variations in the TAG distributions and adding positional information to data from FAMEs analysis which is a relatively new innovation for edible oils (Green et al., 2020; Tu et al., 2016; Wabaidur et al., 2016).
While many chromatographic methods are currently applied using higher cost instruments which require specialist application, these technologies demonstrate potential to differentiate the profiles of a wide range of edible oil types. As is the case for many mass spectrometric technologies, there is much progress in miniaturisation of instruments, providing the future potential for relatively low-cost, rapid and specialist-free point-of-use testing.
The relevance of the sensitivity and dynamic range of the various methods detailed above should be considered in the context of the issue being addressed. Such a cross-analytical technique comparison has not been performed before to our knowledge. In the case of edible oil authenticity, while the absolute sensitivity of methods is not entirely relevant as an oil sample is not limited, the various techniques discussed above will offer different relative dynamic concentration ranges. The required limit of detection (LOD) will likely differ depending on the oil being identified. For example, the required LOD may be lower for oils such as mineral or gutter oil which pose direct and severe safety risks compared to the LOD to determine substitution with botanical origins which differ from the declared origin. The latter scenario facilitates method development using cost-effective and portable technologies which may suffer reduced sensitivity compared to methods which identify particular markers by molecular characterisation.
11. Stakeholder engagement
11.1. Approach to stakeholder engagement
Stakeholders were invited to engage with this project to represent their views to inform FSA. Questionnaires were prepared and agreed with FSA and Defra. Interviews took place based on these questionnaires with government representatives, Official Laboratories, a testing lab in a developing nation, the Food Authenticity Network’s Centres of Expertise (CoEs) (individuals with experience of edible oils testing, representing their respective CoE) and a supply industry representative. The content of these interviews is included in Appendix 1. To maximise levels of engagements with the project by stakeholders in edible oil authenticity, a further online questionnaire was published for 25 working days to offer engagement to stakeholders who were not involved in the interviews described above.
11.2. Conclusions to stakeholder engagement
While conducting the various stakeholder engagement activities involved in this study, it has become increasingly apparent that stakeholders are aware that authenticity fraud is viewed as a risk and that there is a belief throughout the industry that fraudsters are operating in sophisticated manners and are well-informed regarding manners in which to avoid detection of fraud. For this reason, businesses are developing their own testing methods which they are not making public in an attempt to keep a step ahead of the fraudsters. Several supply chain stakeholders spoken to during this study and invited to take part in either video interviews or to engage in the short online survey could not disclose their methods and could engage only at a very minimum level with the project, due to confidentiality requirements regarding their testing approaches. It is apparent that much research and development is constantly underway by stakeholders to develop testing methods aimed at detecting the ever-evolving risks of fraud in the edible oils market. Such development of tests requires much time and investment, aimed at protecting brands, safety and consumer confidence. It is imperative to these companies that their methods are not disclosed to fraudsters who could develop alternative strategies to circumvent detection by these methods. This presents a difficulty when considering sharing methods in the future to provide standardised methods. Future discussion of this issue with the Food Industry Intelligence Network may be of benefit to troubleshoot some possible solutions here.
A summary of the main points arising from the stakeholder engagement is listed below:
-
Fraudsters in edible oil supply are very sophisticated and will work to out-smart testing and overcome regulation.
-
Regulation and guidelines for edible oils, other than olive oils, are severely limited.
-
An agreed sampling and testing strategy, supported by a robust legal framework, would support all stakeholders to ensure oil authenticity.
-
The risks relating to fraud in the supply chain are leading to suppliers working in silos to develop testing methods to prevent fraudsters from learning of these methods via industry networking groups, Codex, etc.
-
The required secrecy of testing methods for edible oil authenticity is delaying or preventing the development of standardised methods.
-
Low-cost, rapid screening methods will be of benefit to strengthen testing in developing nations and to facilitate point-of-use testing in factories across the globe.
-
Stakeholders proposed a two-tier approach to oil authenticity testing where, depending on resources either fatty acid and then sterol testing is conducted, or NIR or Raman is used as the first approach, followed by further confirmatory investigation of suspect samples by mass spectrometry. This would be the recommended approach for Official Laboratories, ideally using rapid, low-cost, point-of-contact spectroscopy methods if developed and validated in the future to analyse high numbers of samples quickly and with minimal cost and minimal training requirements.
-
Having a partner testing laboratory in a well-resourced country like UK would support developing nations in confirmatory testing.
-
There is a critical lack of oil-based reference materials to support quality control in testing.
-
Considerably more collaboration and sharing of intelligence (which is currently viewed as occurring very rarely) would support Official Control in the UK
-
More collaboration and intelligence sharing on an international scale is required due to the long, complex and global nature of the supply chain.
-
More education among suppliers is required regarding the importance of ensuring the authenticity of food, particularly in developing nations.
-
Safety issues regarding Sudan dyes and the need for more strict regulation were also raised.
-
Education of consumers regarding their expectations of the colour of palm oil may reduce the temptation for fraudsters to adjust the colour using toxic Sudan dyes.
-
Increased budgets are required across testing labs to support sampling, surveys, including budgets for confirmatory screening tests.
-
Gutter oil is a safety issue with a current outbreak of gutter oil in Asia beginning to spread to other continents.
-
It would be of benefit to source a high number of authentic oil samples, taking into account different botanical origins, different refining levels, geographical origin etc, and making these available for testing and modelling.
12. Final Project Conclusions and Recommendations
12.1. Final Conclusions
The HorizonScan™ data discussed in this report corroborate the findings of the OPSON X report of 2021, highlighting that adulteration of edible oils is a concern for the global supply chain. Trends in the increasing incidence of safety risks such as reports of mineral oil and Sudan dye being found in edible oils is a serious safety concern. Adulteration reports also exhibit traceability risks in food supply which are damaging to consumer trust, safety and the food economy.
While regulation exists for the authenticity of olive oils, regulation is lacking for other edible oils. Much effort has been made to develop tests to authenticate olive oils, mainly by the producing nations and in order to protect their economies due to the relative high value of olive oil. Certain of these methods have been standardised and are managed by International Olive Council, Codex Alimentarius and International Organisation for Standardisation. For other edible oils however, while research and development has been undertaken, the large studies incorporating a wide range of oil types sourced over a range of suppliers and of seasons required is lacking. Until this occurs, the standardisation of an approach cannot be considered. Regulation would ideally define what ‘authentic’ looks like, stating parameters to measure and permitted tolerances and defining the methods to use which would have gone through a collaborative trial. These methods would then be applied to support surveillance activities.
The literature review highlighted that methods which have been investigated to test the authenticity of edible oils range from screening methods with a point-of-contact ambition, often based on spectroscopic technologies, to methods aimed at confirmation, including the profiling and/or marker detection of chemical components including fatty acids, sterols and triacylglycerols, sometimes to differentiate a wide variety of botanical origin of oilseed. However, the publications represent methods that are in their relative infancy, often not having been tested on a representatively wide range of oils sourced from a range of suppliers or sources, not accounting for natural and seasonal variation and having not undergone collaborative trials. The lack of uptake in international proficiency testing schemes demonstrates a concerning lack of engagement and a lack of accuracy of the tests used.
An issue that became increasingly apparent throughout the project was that this authenticity risk to the edible oil industry is such that businesses are working in silos to develop testing methods which they do not disclose. An outcome of the various stakeholder engagement interviews demonstrated that this is due to concerns of the risk of fraudsters becoming informed of testing approaches and adopting practices to avoid detection by these approaches. Fraud within the edible oils industry is considered to be highly sophisticated with criminals and the industry constantly evolving their approaches in order to keep one step ahead of each other. There is a clear concern that fraudsters may be highly informed regarding the chemistry of oils and the testing technologies available to identify fraud. We conjecture that this lack of sharing of research data is a major barrier to method development and the associated standardisation of methods which inherently requires sharing of methods. There is a lack of trust throughout the industry due to the sophisticated approach by fraudsters and it is considered to be against the interest of stakeholders to share details of the methods being applied to oil testing.
A concerning lack of interest in participating in proficiency trials was highlighted. Of the low number of participants who joined the three authenticity PTs which it has been viable to run, the accuracy of the authenticity testing was also a concern. A lack or absence of matrix-matched reference materials was highlighted throughout the project with the only edible oil authenticity reference materials identified as available to purchase being those produced during these Fapas® edible oil authenticity PT rounds. Investment is therefore required to prepare certified reference materials of oils and oil mixtures for a broad range of oil types. Sourcing pure and authentic material to make CRMs can be a challenging task. Refined vegetable oils and fats are available in high volume, and the overall content may have originated from different origins, periods of production and suppliers are often not transparent (not willing to be transparent) and cross-contamination can also occur. Support is needed to source authentic oil samples as CRMs.
This report makes recommendations for the future to support consumers, the edible oils industry and the associated UK economy into the future. Investment is required to develop a point-of-use screening method and also a confirmatory method, tested on a wide range of oil botanical types, suppliers and geographies and across a range of growing seasons to account for natural variability. Spectroscopy approaches such as Raman or FTIR offer much potential for rapid, low-cost point-of-use screening. Such methods could support OLs in the rapid and low-cost testing of large numbers of oil samples. This methodology requires minimal training and would support surveillance exercises, offering a rapid, low-cost test rather than time-consuming FAMEs analysis which involves time-consuming derivatisation and specialist analysis and data interpretation by skilled personnel.
Emerging technologies worthy of future investigation include Direct Analysis in Real Time (DART) and Atmospheric pressure Solids Analysis Probe (ASAP). Both show promise for robust measurement and DART analysis is already showing potential to determine food and illegal drugs. High resolution mass spectrometry to investigate the position of fragmentation within a molecule may identify oil-specific markers and signatures offers a promising starting point for initial discovery work. Lipidomics using Fourier Transformation (FT) mass spectrometry to investigation double bonds type and position in lipids is an interesting area for future focus to differentiate the species of an oil.
A major advance in oil testing over recent years with the potential to support testing in OLs in the future is the ability to quickly and accurately assess the triglyceride composition of oils. Triglycerides, or triglycerols (TAGs) are the major lipid component of edible oils. Modern LC-MS based approaches can provide data to characterise and identify the structure of TAGs providing data regarding; the length of the fatty acid chains, the number and position of double bonds on those fatty acid chains, and importantly, the position at which the fatty acid chains are attached to the glycerol backbone. The TAG composition of oils has been shown to vary significantly between oils from different botanical sources and this innovation should be further explored though method validation to understand method uncertainty. Such a method could later be transferred onto lower tech platforms such as benchtop LC-MS. Investment is required to further develop these methods which show the potential in edible oil authenticity determinations. The identification of oils is an issue in the methods used in Official Laboratories if more than one oil type is present. The detection of botanical type-specific markers would mitigate this issue. The aim would be to identify TAG markers which Official Laboratories could detect using their existing liquid chromatography triple quadrupole mass spectrometry instruments, currently used by many OLs for other applications such as veterinary medicine and pesticide detection in food. Indeed, Fera Science has trained several of the Official Laboratories to use their available triple quadrupole instruments in other authenticity areas such as the species origin determination of highly processed meats and gelatine using cross-government funding via the Joint Knowledge Transfer Programme. This marker analysis approach has the added potential for analysis of oil authenticity in composite final products rather than in only samples of liquid oil. The analysis of DAGs and TAGs may also prove valuable to determine species specificity after cooking due to degradation profiles.
Education in the food supply chain regarding the safety risks of Sudan dyes, mineral oil and re-used oils (so-called gutter oils) may reduce the number of such adulteration incidents involving these toxic materials. The education of consumers and suppliers alike regarding the expected colour of palm oils may reduce the number of incidents in which toxic Sudan dyes are applied to adjust oil colour as this knowledge may reduce the demand for the colour-adjusted oil.
Once suitable testing approaches are developed, some form of method challenge must take place with an ideal aim to produce standardised methods. At present, collaboration is not occurring widely, in a bid to prevent counterfeiters from becoming aware of the latest testing capabilities. Issues relating to the avoidance of sharing of methods could be overcome to a certain extent: in the absence of the possibility of an inter-laboratory collaborative trial which would involve sharing knowhow between businesses and testing labs, a single institute with a range of expertise and instrument types could act to curate and challenge the approaches. Then, in an intra-laboratory trial, the method could be performed and validated as far as possible by a range of analysts at the institute on a range of different suitable instruments, each underpinned by the required technology.
The development of standardised methods would facilitate the development of the regulations, with sampling and testing regime guidance. Increased budgets for testing laboratories would then support oil authenticity in the supply chain. However, as discussed in detail above, standardisation and transparency can inform fraudsters of testing capabilities, driving them to develop new kinds of edible oil counterfeiting to circumvent detection by testing, so the solution is not straightforward.
Increased sharing of data between the private sector and testing labs when issues arise in the supply chain would support Official Labs in prioritising testing.
12.2. Evidence-based recommendations to support enforcement
The following recommendations are made, based on the evidence gathered throughout this study, to support Official Laboratories in ensuring that edible oils are authentic:
-
Invest in method development for verifying the authenticity of edible oils.
-
Investment is required to validate these methods on oils from a wide range of oil botanical types, suppliers and geographies and across a range of growing seasons to account for natural variability and for mixing of same-species oils during production. Spectroscopy such as FTIR and Raman offers much potential for rapid, low-cost point-of-use screening. Such rapid and low-cost testing would support OLs in the rapid testing of large sample numbers to support surveillance exercises.
-
Invest in confirmatory testing which is accessible to Official Laboratories to test suspect or unsatisfactory oils identified during the rapid screening process. LC-MS triple quadrupole identification of botanical origin-specific TAG markers shows potential and would be accessible to public analysts OLs who already use triple quadrupole LC-MS to test for presence of veterinary drugs, pesticides, etc. Analysis of TAGs also supports issues currently highlighted by OLs when complications arise to identify oil types in a mixture of botanical origins by current methods. Testing of TAGs may also support the future analysis to authenticate oils present in composite products so may not be applicable only to analysis of liquid oil samples.
-
Develop guidelines, and later, regulation for the composition and testing for the authenticity of edible oils. While regulation exists for the authenticity of olive oils, it is lacking for other edible oils. This will support the testing of edible oils, including the future standardisation of methods.
-
Development of standardised methods would facilitate the development of the regulations, with sampling and testing regime guidance.
-
Once methods have been validated and standardised, this should support uptake in proficiency testing.
-
Investment is required to prepare a wide range of matrix-matched (i.e. oil-based) Certified Reference Materials (CRMs) of oils and oil mixtures for a broad range of oil types for QC purposes.
-
Regulation would provide testing parameters and tolerances which OLs could interrogate when testing. A starting point for the content of the regulation could be consideration of existing Codex Alimentarius guidelines for edible oils but preparation of regulations is likely to be a long, step-by-step process with the regulation becoming more detailed only when testing methods are developed and validated to underpin authenticity.
-
Regulation, standardisation of testing methods and certified reference materials would provide OLs with greater confidence in testing data.
-
Education of consumers regarding the expected colouration of palm oil to negate the current practice by fraudsters to colour-adjust palm oils using toxic Sudan dyes.
-
Educate naïve fraudsters in the supply chain regarding the severe health risks of using non-food-grade oils such as mineral and gutter oils and toxic Sudan dyes to fraudulently adulterate food oils to help to prevent these issues in the future. If counterfeiters were aware that this is a safety issue and that it attracts much attention regarding identification later in the supply chain, the practice may quickly be reduced.
13. Appendix 1. Stakeholder interview transcripts
Please refer to supplementary file Appendix 1.





_results_as_a_kernel_density_plot.png)
_results_as_a_kernel_density.png)

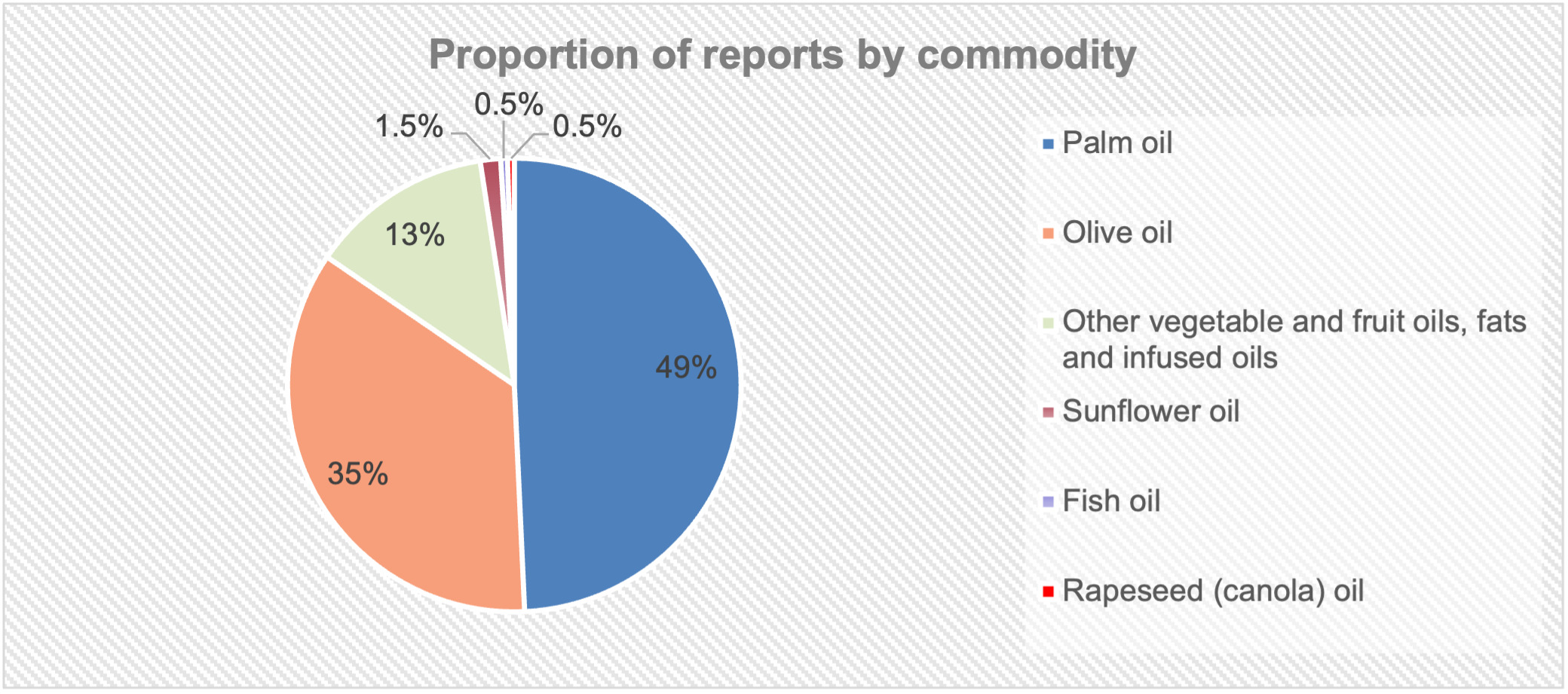
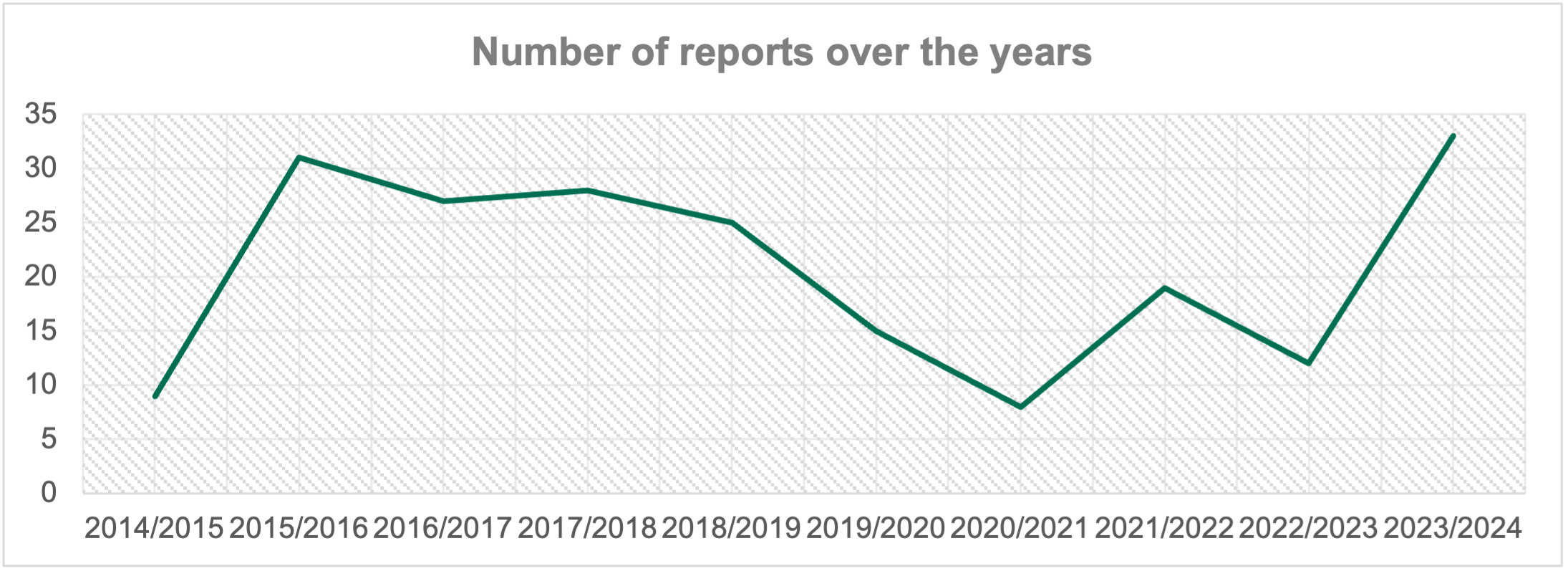
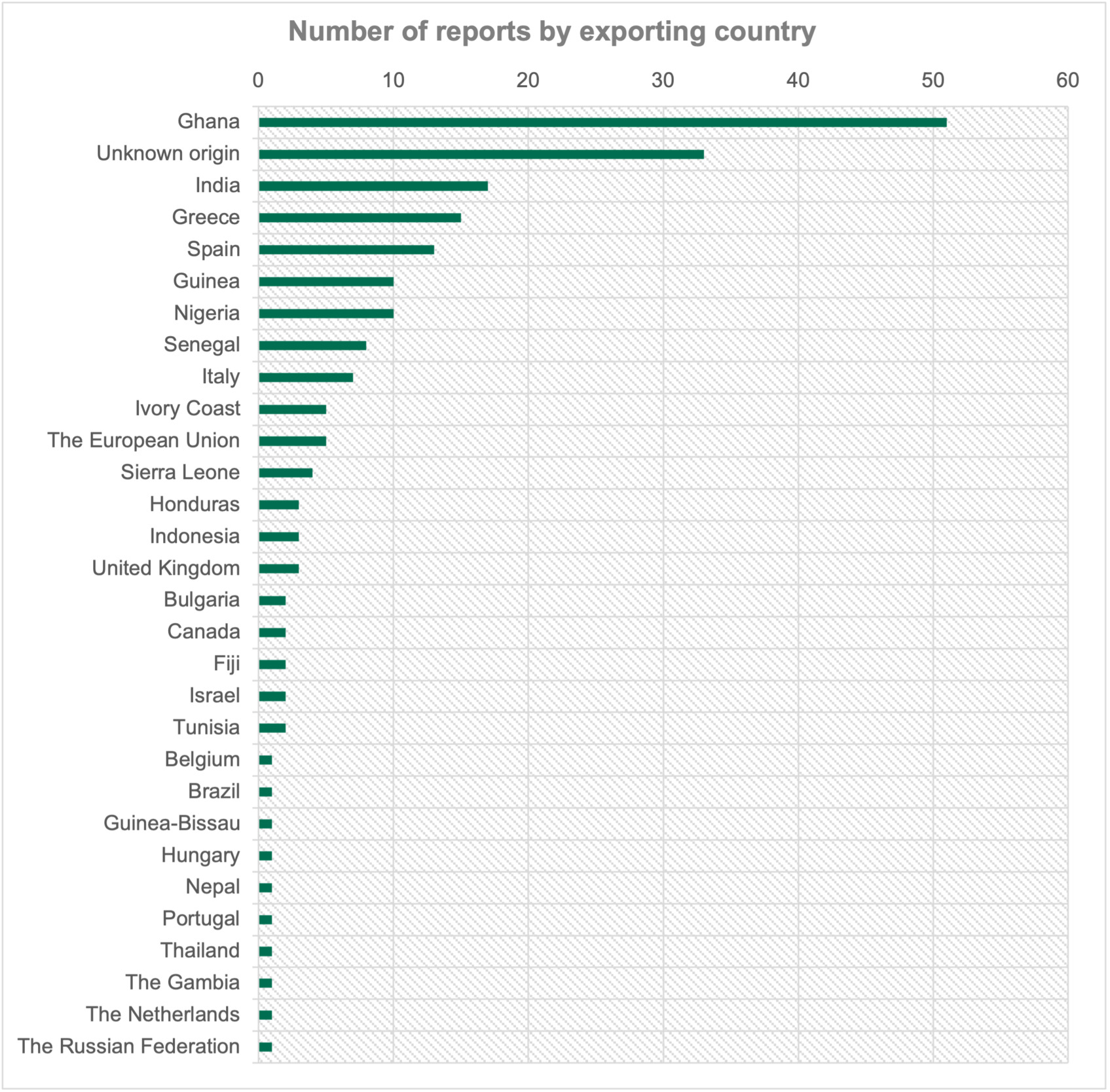


_results_as_a_kernel_density_plot.png)
_results_as_a_kernel_density.png)